What Is The Ph Of A 6 50X10 3 M Koh Solution
What Is The Ph Of A 6 50X10 3 M Koh Solution - Calculate the theoretical ph value of the koh solution. Steps to find ph of koh solution. We'll begin by writing the balanced dissociation equation for koh. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. In the case of a strong base like koh, it. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. What is the ph of this solution? Next, we shall determine the poh of the solution. State whether the given solution is acidic, basic or.
The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. We'll begin by writing the balanced dissociation equation for koh. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. State whether the given solution is acidic, basic or. Steps to find ph of koh solution. Next, we shall determine the poh of the solution. In the case of a strong base like koh, it. Calculate the theoretical ph value of the koh solution. What is the ph of this solution?
To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. Steps to find ph of koh solution. Calculate the theoretical ph value of the koh solution. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. What is the ph of this solution? We'll begin by writing the balanced dissociation equation for koh. State whether the given solution is acidic, basic or. Next, we shall determine the poh of the solution. In the case of a strong base like koh, it.
At 90 ^o C, the pH of 0.001M KOH solution will be
Calculate the theoretical ph value of the koh solution. What is the ph of this solution? We'll begin by writing the balanced dissociation equation for koh. In the case of a strong base like koh, it. Steps to find ph of koh solution.
Solved 1. What is the pH of a 6.50 x 103 M KOH solution?
What is the ph of this solution? Steps to find ph of koh solution. We'll begin by writing the balanced dissociation equation for koh. Calculate the theoretical ph value of the koh solution. State whether the given solution is acidic, basic or.
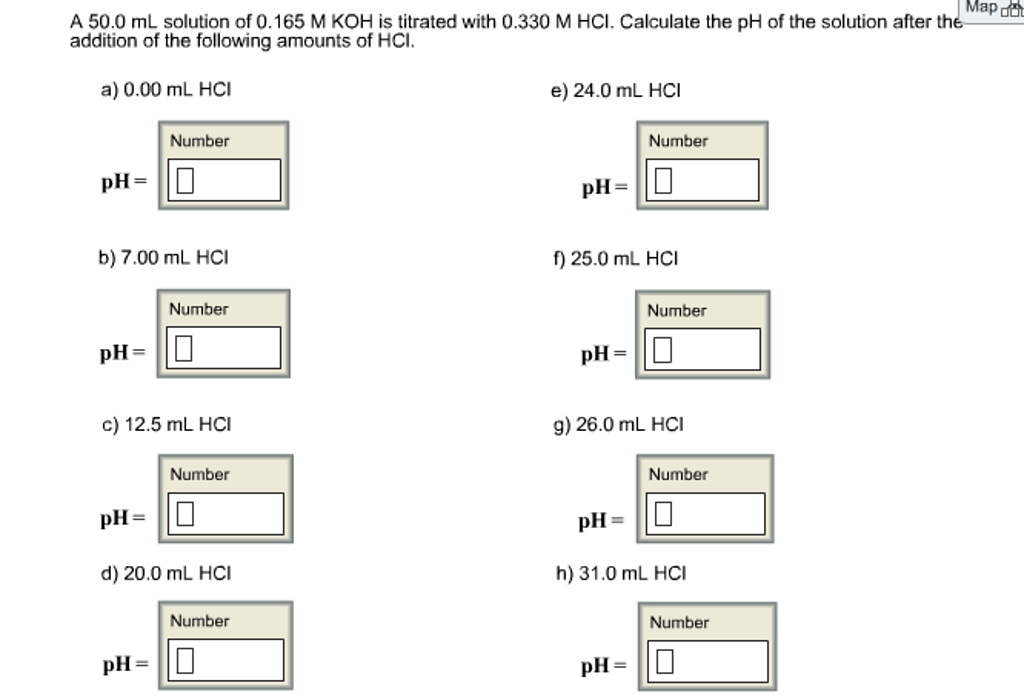
Solved A 50.0 mL solution of 0.165 M KOH is titrated with
To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. What is the ph of this solution? We'll begin by writing the balanced dissociation equation for koh. State whether the given solution is acidic, basic or. Calculate the theoretical ph value of the koh solution.
SOLVED determine the pH of a 0.04 M KOH solution
The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. Calculate the theoretical ph value of the koh solution. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. Next, we shall determine the poh of the solution. What is the ph of this solution?
SOLVED Calculate the pH of a 2.8 x 10^3 M KOH solution.
Calculate the theoretical ph value of the koh solution. State whether the given solution is acidic, basic or. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. Next, we shall determine the poh of the solution. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the.
SOLVED Calculate the pH of 100.00 mL of 0.10 M KOH solution after 145.
Steps to find ph of koh solution. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. State whether the given solution is acidic, basic or. We'll begin by writing the balanced dissociation equation for koh. What is the ph of this solution?
Solved Determine the pH in a 2.58 x 103 M KOH solution at
We'll begin by writing the balanced dissociation equation for koh. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. What is the ph of this solution? Steps to find ph of koh solution. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m.
Solved 1. What does the pH of a solution tell you? 2. What
Next, we shall determine the poh of the solution. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. State whether the given solution is acidic, basic or. In the case of a strong base like.
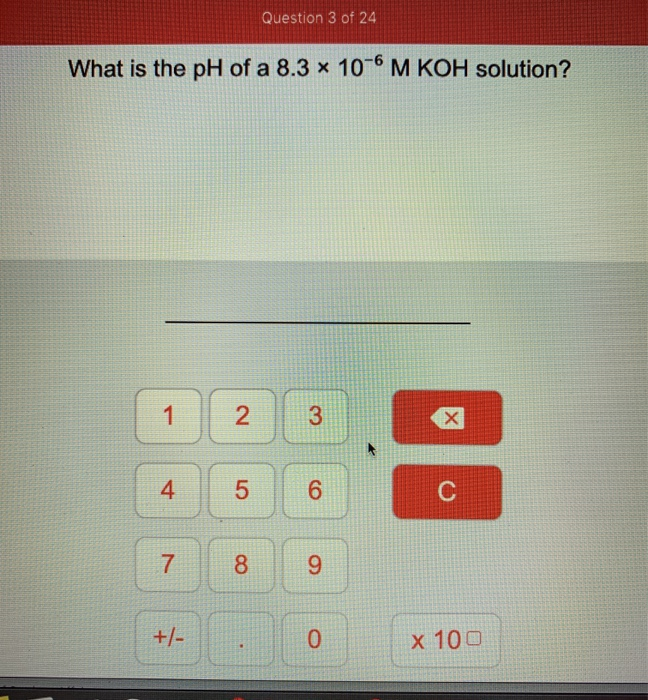
Solved what is the pH of a 8.3 x 10^6 M KOH solution
State whether the given solution is acidic, basic or. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the solution. Steps to find ph of koh solution. Calculate the theoretical ph value of the koh solution. Next, we shall determine the poh of the solution.
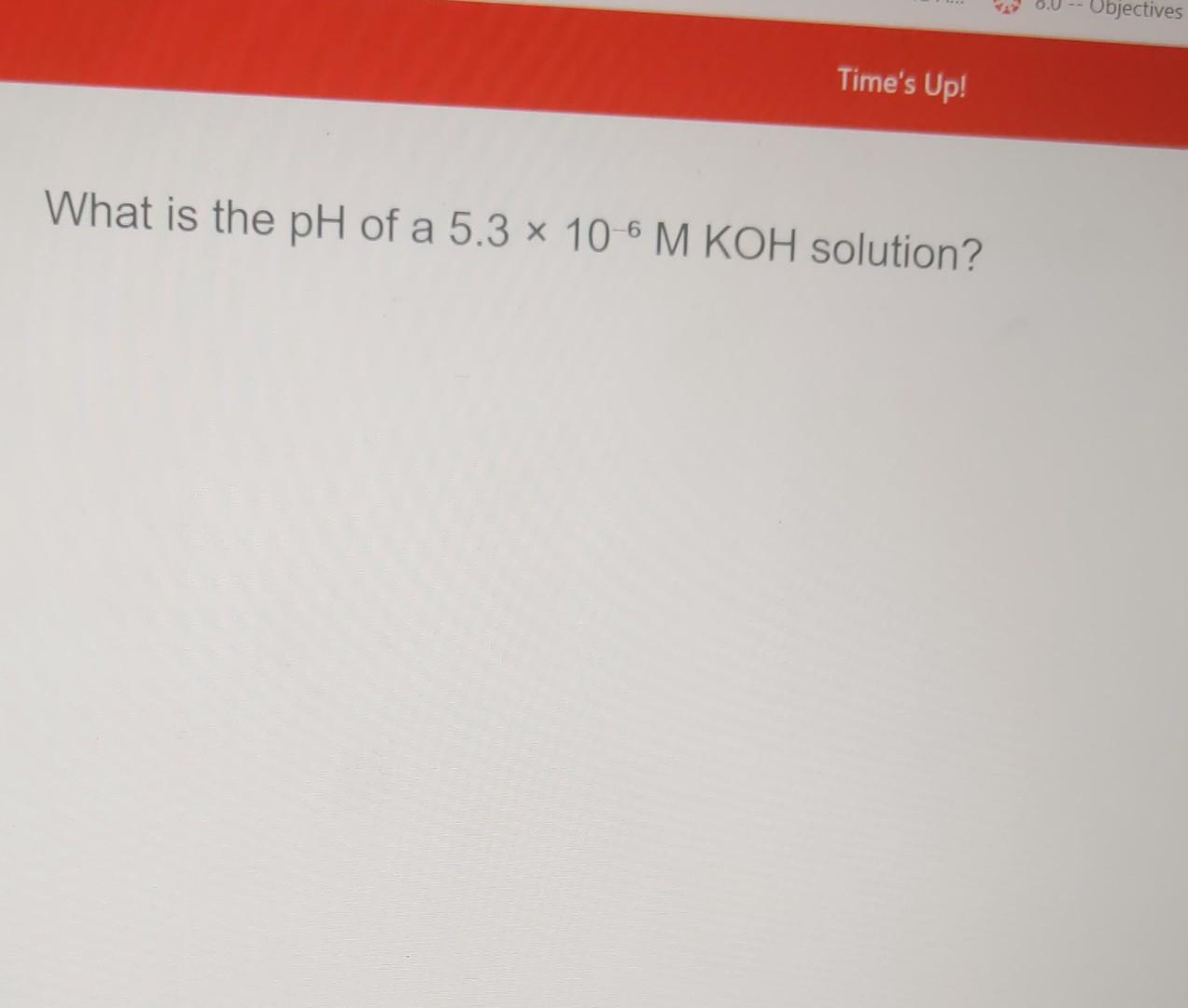
Solved What is the pH of a 5.3×10−6MKOH solution?
Next, we shall determine the poh of the solution. State whether the given solution is acidic, basic or. The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. We'll begin by writing the balanced dissociation equation for koh. To find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in.
Next, We Shall Determine The Poh Of The Solution.
Steps to find ph of koh solution. State whether the given solution is acidic, basic or. We'll begin by writing the balanced dissociation equation for koh. What is the ph of this solution?
To Find The Ph Of A Solution, We Need To Determine The Concentration Of Hydrogen Ions (H+) In The Solution.
The hydroxide ion concentration of a solution is 1.0 × 10 − 9 m. Calculate the theoretical ph value of the koh solution. In the case of a strong base like koh, it.