What Is The Ph Of A 0 6 M Hno3 Solution
What Is The Ph Of A 0 6 M Hno3 Solution - Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ) + h 2 o ( l ) → h 3 o + + n o 3 − and so p h = −. What is ph of the solution obtained by mixing 10. Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? The ph of a 0.6 m hno3 solution is approximately 0.23. The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. These online calculators calculate the ph of a solution. The ph of any solution can be calculated by the formula:. This is the required answer.
What is ph of the solution obtained by mixing 10. This is the required answer. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ) + h 2 o ( l ) → h 3 o + + n o 3 − and so p h = −. The solution of a strong acid is completely ionized. Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? The ph of any solution can be calculated by the formula:. The ph of a 0.6 m hno3 solution is approximately 0.23. These online calculators calculate the ph of a solution. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid.
The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. The ph of a 0.6 m hno3 solution is approximately 0.23. What is ph of the solution obtained by mixing 10. The solution of a strong acid is completely ionized. These online calculators calculate the ph of a solution. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ) + h 2 o ( l ) → h 3 o + + n o 3 − and so p h = −. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. This is the required answer.
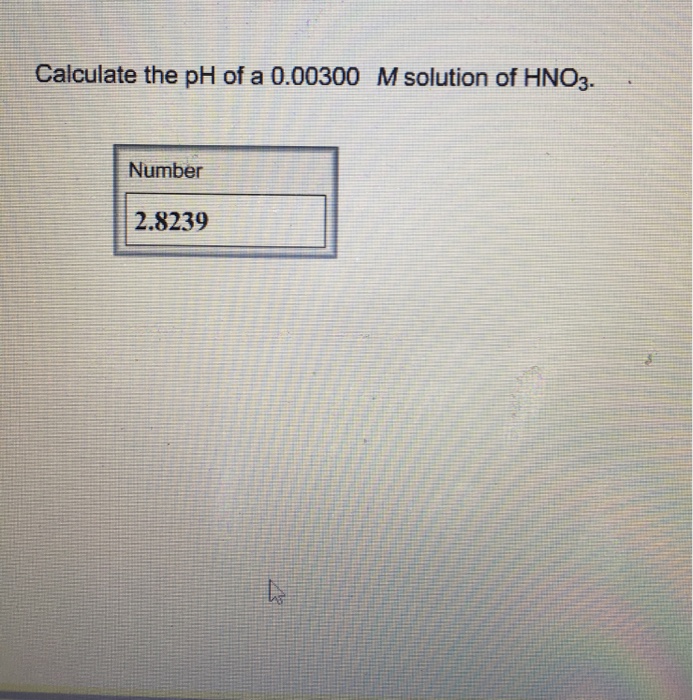
Solved Calculate the pH of a 0.00300 M solution of HNO_3.
This is the required answer. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. The solution of a strong acid is completely ionized. The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)?
SOLVED Calculate the pH for a 0.348 M HNO3 solution.
Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. This is the required answer. What is ph of the solution obtained by mixing 10. The solution of a strong acid is completely ionized.
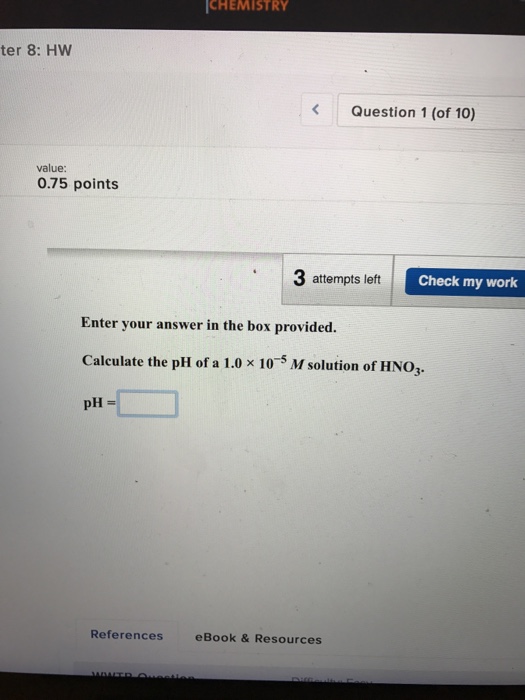
Solved Calculate the pH of a 1.0 times 10^5 M solution of
Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ) + h 2 o ( l ) → h 3 o + + n o 3 −.
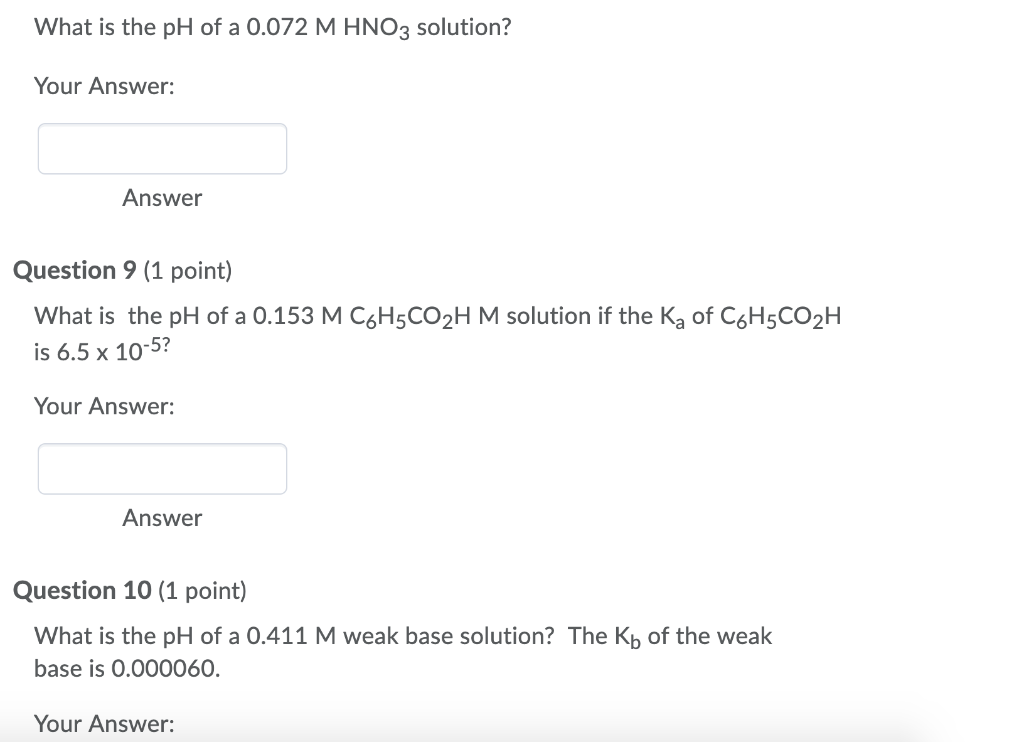
Solved What is the pH of a 0.072 M HNO3 solution? Your
What is ph of the solution obtained by mixing 10. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. The ph of any solution can be calculated by the formula:. These online calculators calculate the ph of a solution. This is because nitric acid is a strong acid that completely ionizes in solution,.
Solved Determine the pH of a 0.033 M HNO3 solution. O 6.26 O
These online calculators calculate the ph of a solution. This is the required answer. The solution of a strong acid is completely ionized. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. What is ph of the solution obtained by mixing 10.
28.The pH of solution formed by mixing 40 ml of 0.10 M HCl and 10 ml of
The ph of any solution can be calculated by the formula:. The solution of a strong acid is completely ionized. The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3.
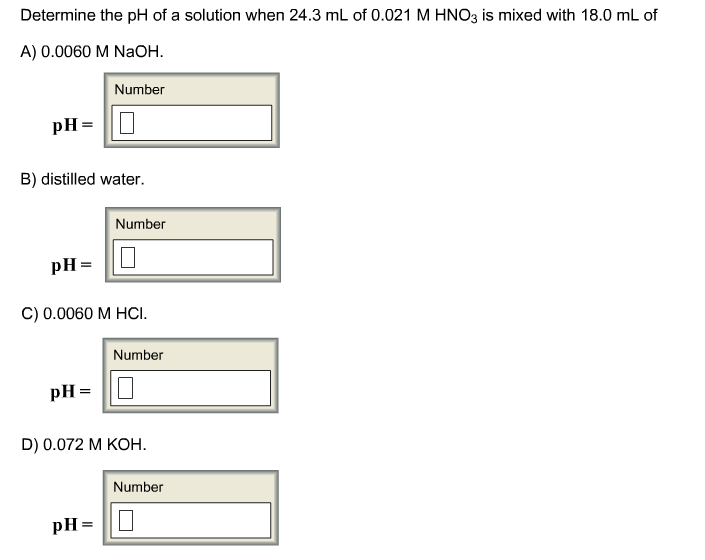
Solved Determine the pH of a solution when 24.3 mL of 0.021
The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. The solution of a strong acid is completely ionized. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. These online.
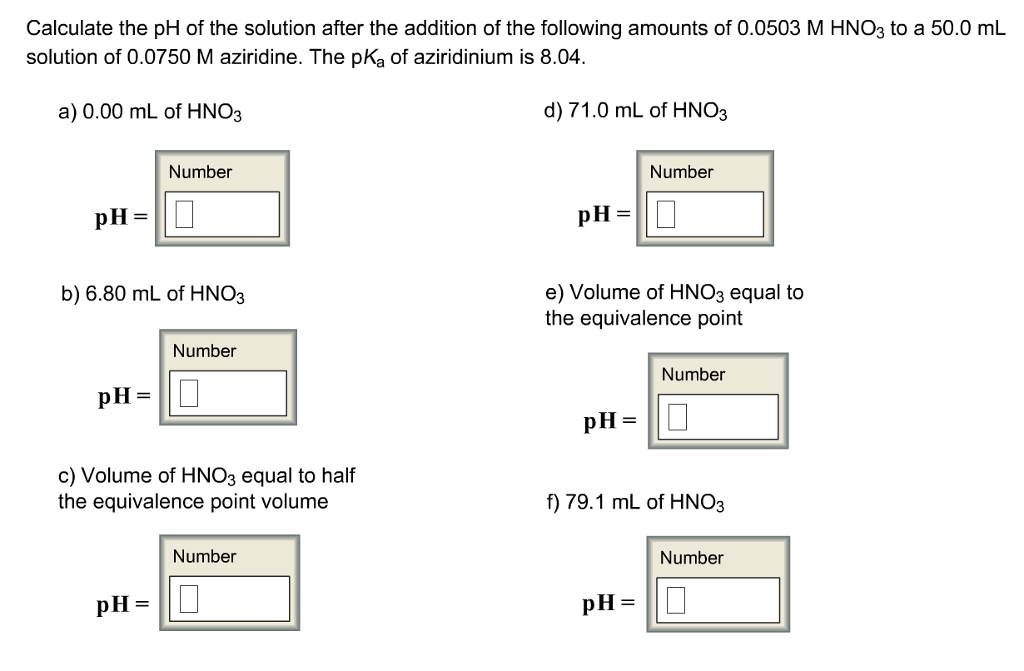
Solved Calculate the pH of the solution after the addition
Calculate ph and poh of the solution containing 0.1m of h3po4 (pka1=2.12, pka2=7.21, pka3=12.67)? The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. The ph of a 0.6 m hno3 solution is approximately 0.23. By definition,.
45+ calculate the ph of a 0.0150 m hno3 solution RaffeRadison
What is ph of the solution obtained by mixing 10. This is the required answer. Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ).
Solved 2. pH and pOH problems. What is the pH of a 0.01 M
These online calculators calculate the ph of a solution. This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. The solution of a strong acid is completely ionized. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o.
The Solution Of A Strong Acid Is Completely Ionized.
Calculate the ph of a solution with 1.2345 × 10 − 4 mhcl, a strong acid. What is ph of the solution obtained by mixing 10. These online calculators calculate the ph of a solution. By definition, p h = − lo g 1 0 [h 3 o +] we assume that nitric acid dissociates quantitatively h n o 3 ( a q ) + h 2 o ( l ) → h 3 o + + n o 3 − and so p h = −.
Calculate Ph And Poh Of The Solution Containing 0.1M Of H3Po4 (Pka1=2.12, Pka2=7.21, Pka3=12.67)?
This is because nitric acid is a strong acid that completely ionizes in solution, resulting in a high. The ph of a 0.6 m hno3 solution is approximately 0.23. The ph of 0.6 m nitric acid is 0.222 which is a highly acidic solution. This is the required answer.