What Is The Ph Of A 0 001 M Koh Solution
What Is The Ph Of A 0 001 M Koh Solution - This concentration is crucial for further calculations, such as determining the ph of the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the.
At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. This concentration is crucial for further calculations, such as determining the ph of the.
This concentration is crucial for further calculations, such as determining the ph of the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the.
What is the pH of a .001 M solution of HCl? Socratic
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m.
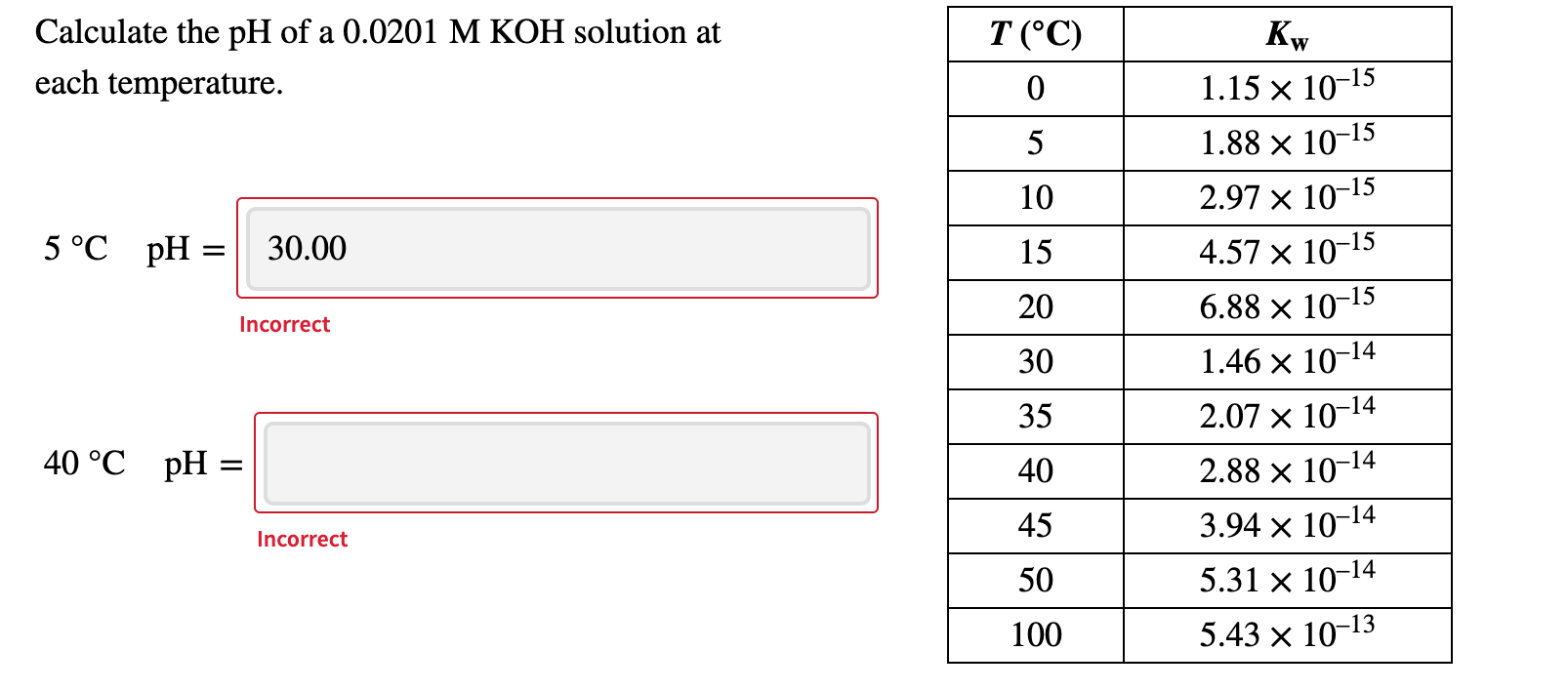
Solved T(°C) Calculate the pH of a 0.0201 M KOH solution at
This concentration is crucial for further calculations, such as determining the ph of the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be:.
SOLVED determine the pH of a 0.04 M KOH solution
This concentration is crucial for further calculations, such as determining the ph of the. The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml.
SOLUTION Calculate the pH of a 0 58 M KOH solutionSolutionpOH = log(0
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m.
[ANSWERED] What is the theoretical pH of a 0 001 M HNO3 solution The pH
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. This concentration is crucial for further calculations, such as determining the ph of the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml.
Solved Find the pH of each solution 0.010 M HBr 0.010 M
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. This concentration is crucial for further calculations, such as determining the ph of the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml.
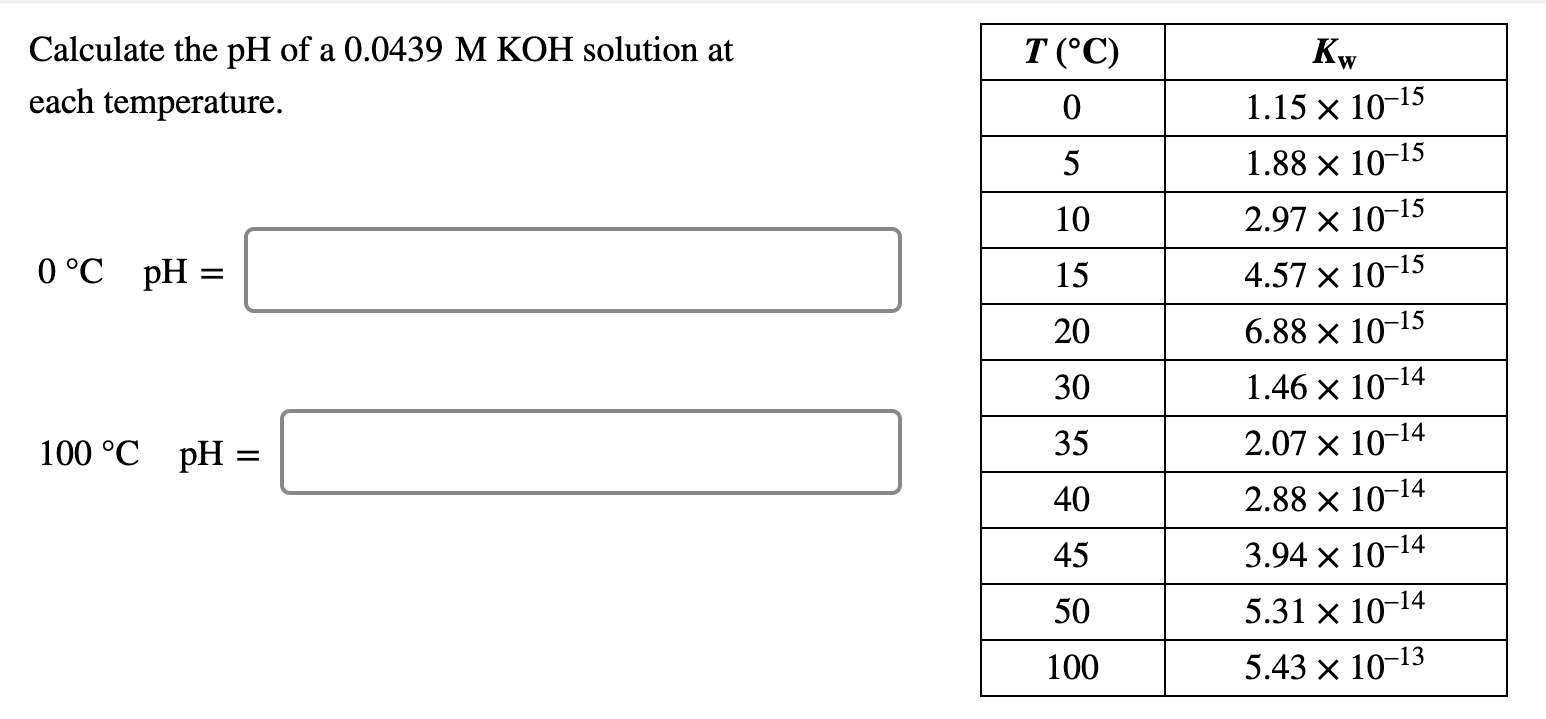
Solved Calculate the pH of a 0.0439 M KOH solution at each
At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: This concentration is crucial for further calculations, such as determining the ph of the..
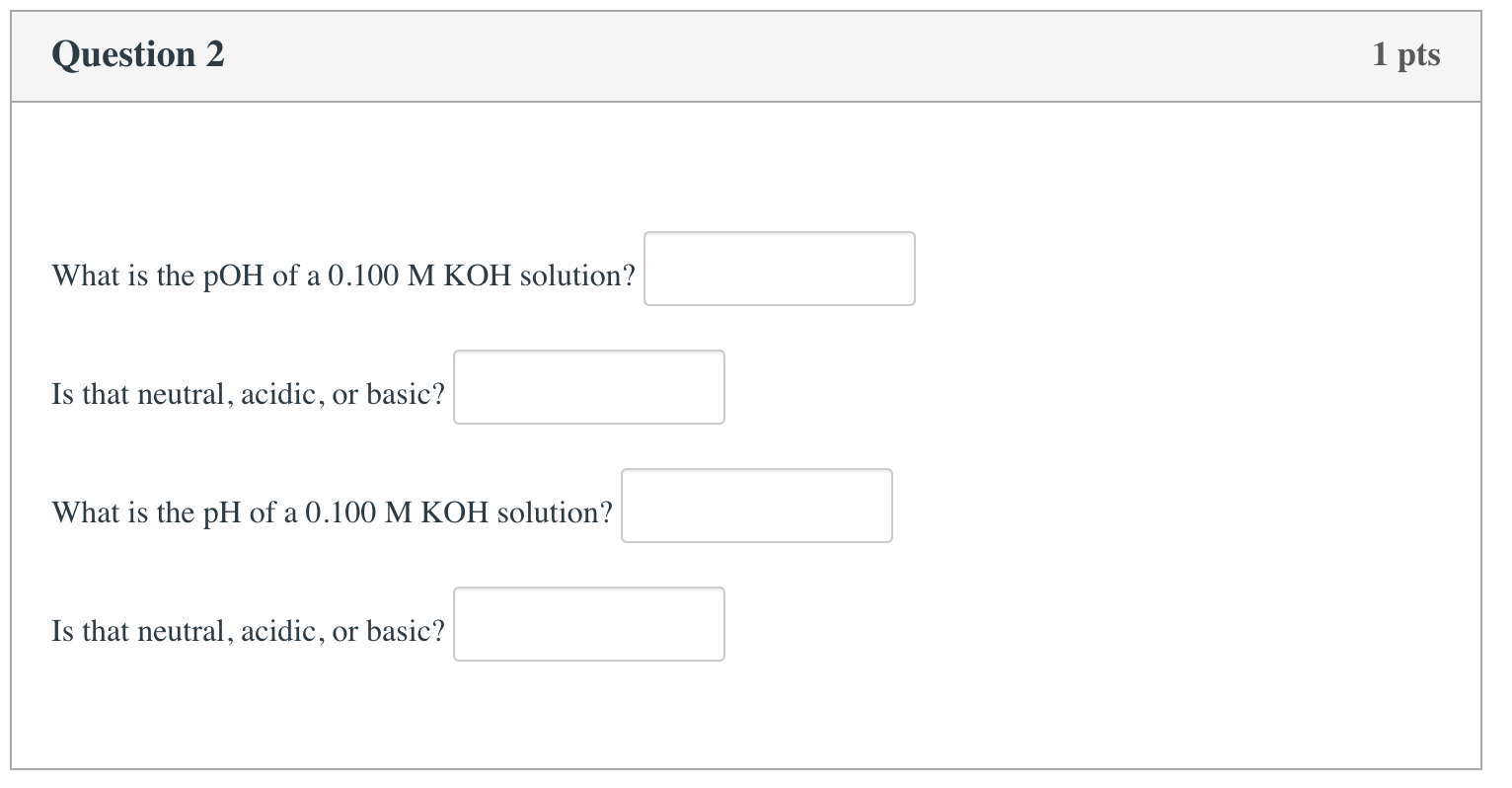
Solved Calculate the pH of 0.01 M KOH solution. (KOH is
At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be: The ph of a 0.001 m koh solution is 14 to find the ph.
At 90 ^o C, the pH of 0.001M KOH solution will be
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m.
This Concentration Is Crucial For Further Calculations, Such As Determining The Ph Of The.
The ph of a 0.001 m koh solution is 14 to find the ph of a solution, we need to determine the concentration of hydrogen ions (h+) in the. At 90 o c, pure water has [h +] = 10 − 6 m, if 100 ml of 0.2 m h c l is added to 200 ml of 0.1 m k o h at 90 o c then ph of the resulting solution will be:


![[ANSWERED] What is the theoretical pH of a 0 001 M HNO3 solution The pH](https://media.kunduz.com/media/sug-question-candidate/20220513044634181988-4440783.jpg?h=512)