What Is The Molecular Shape Of If5
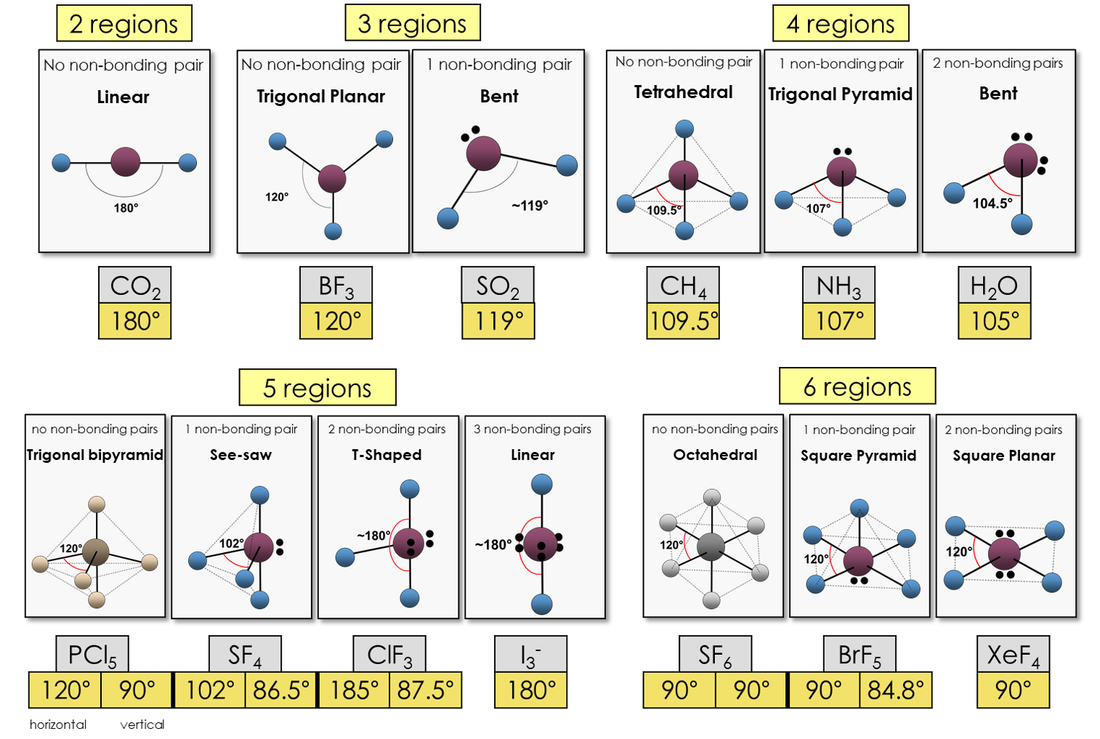
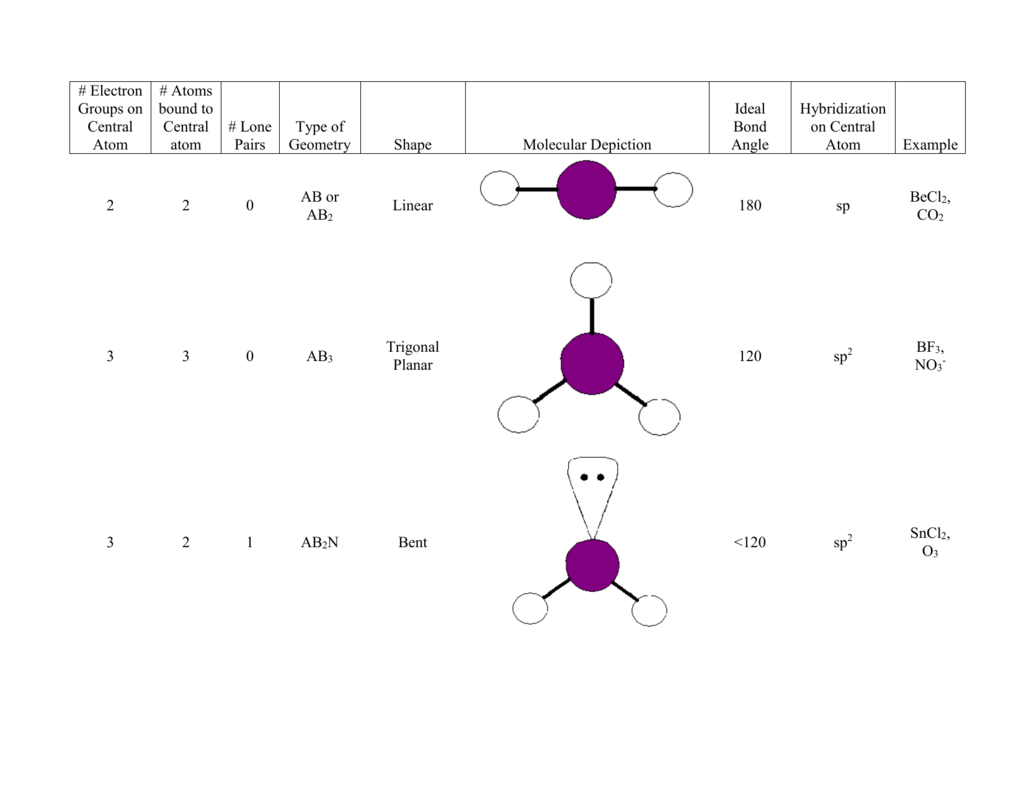
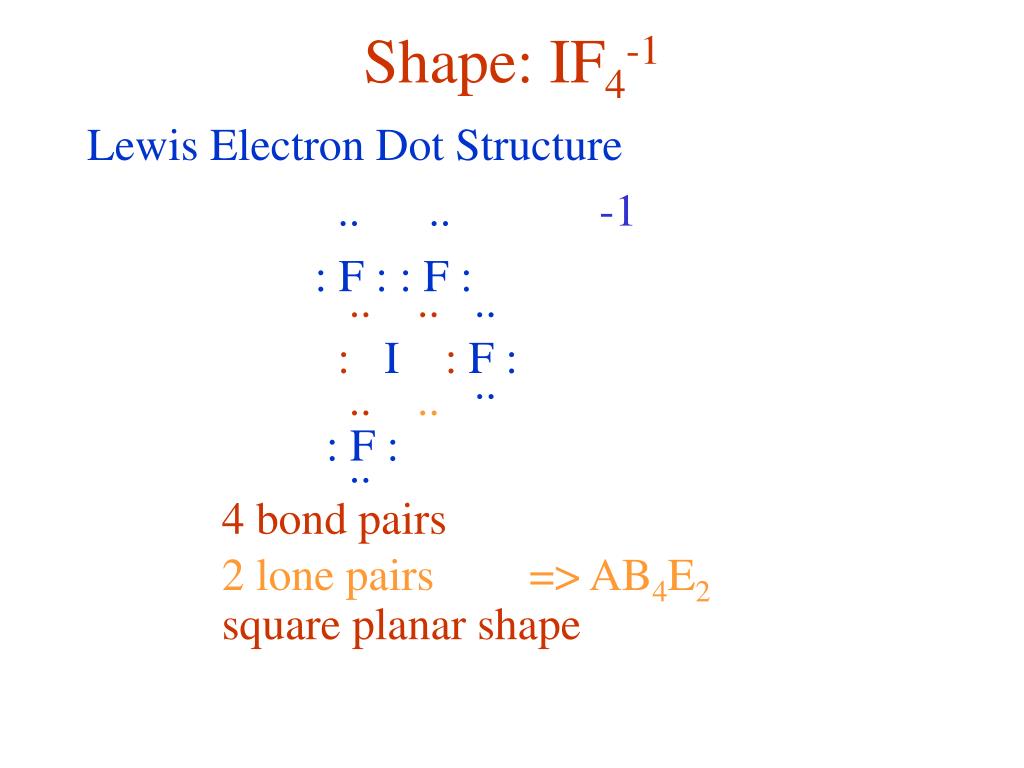
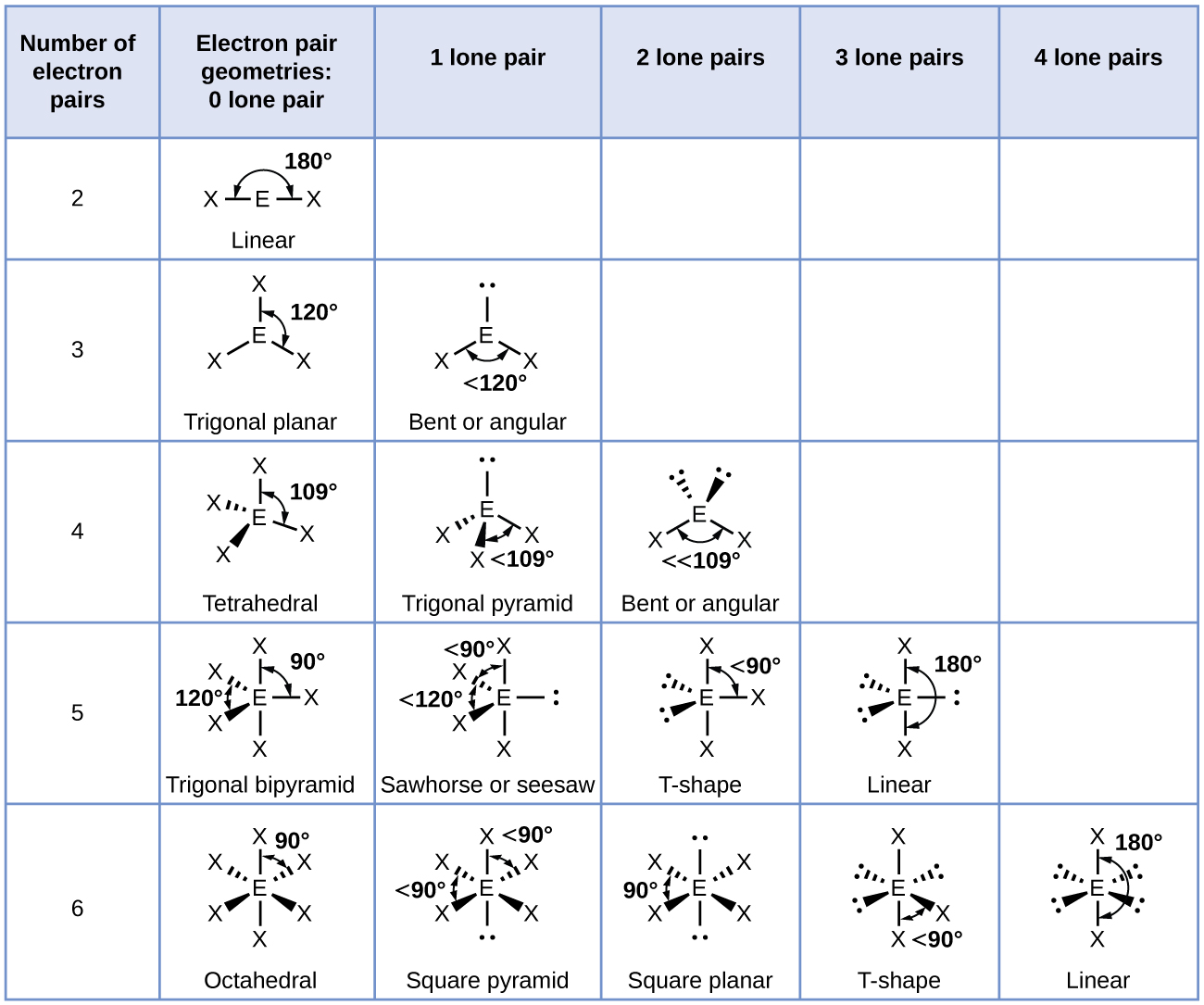
What Is The Molecular Shape Of If5 - A=central atom, x= numbers of atoms bonded to. Xef 4 molecule is square planar in shape. Vsepr theory explained that if5 is ax 5 e type molecule. A lone pair of electrons present on the central iodine atom in if. The molecular geometry or shape of the if 5 molecule is square pyramidal. If 5 lewis structure shape.
A lone pair of electrons present on the central iodine atom in if. Xef 4 molecule is square planar in shape. Vsepr theory explained that if5 is ax 5 e type molecule. If 5 lewis structure shape. A=central atom, x= numbers of atoms bonded to. The molecular geometry or shape of the if 5 molecule is square pyramidal.
If 5 lewis structure shape. A=central atom, x= numbers of atoms bonded to. A lone pair of electrons present on the central iodine atom in if. Xef 4 molecule is square planar in shape. Vsepr theory explained that if5 is ax 5 e type molecule. The molecular geometry or shape of the if 5 molecule is square pyramidal.
4. Molecular Shapes
Xef 4 molecule is square planar in shape. A=central atom, x= numbers of atoms bonded to. If 5 lewis structure shape. Vsepr theory explained that if5 is ax 5 e type molecule. The molecular geometry or shape of the if 5 molecule is square pyramidal.
The Geometrical Arrangement Of Electrons And Molecular
Xef 4 molecule is square planar in shape. A=central atom, x= numbers of atoms bonded to. Vsepr theory explained that if5 is ax 5 e type molecule. A lone pair of electrons present on the central iodine atom in if. The molecular geometry or shape of the if 5 molecule is square pyramidal.
If5 Molecular Geometry ma
If 5 lewis structure shape. The molecular geometry or shape of the if 5 molecule is square pyramidal. A=central atom, x= numbers of atoms bonded to. Xef 4 molecule is square planar in shape. Vsepr theory explained that if5 is ax 5 e type molecule.
IF5 Lewis structure, molecular geometry, bond angle, hybridization
If 5 lewis structure shape. A lone pair of electrons present on the central iodine atom in if. A=central atom, x= numbers of atoms bonded to. Vsepr theory explained that if5 is ax 5 e type molecule. Xef 4 molecule is square planar in shape.
PPT Molecular Geometry and Chemical Bonding Theory PowerPoint
A lone pair of electrons present on the central iodine atom in if. The molecular geometry or shape of the if 5 molecule is square pyramidal. A=central atom, x= numbers of atoms bonded to. If 5 lewis structure shape. Vsepr theory explained that if5 is ax 5 e type molecule.
5.2 Molecular Shape Chemistry LibreTexts
A lone pair of electrons present on the central iodine atom in if. If 5 lewis structure shape. Xef 4 molecule is square planar in shape. A=central atom, x= numbers of atoms bonded to. Vsepr theory explained that if5 is ax 5 e type molecule.
Electron domain geometry vs molecular shape chart guglafro
Vsepr theory explained that if5 is ax 5 e type molecule. The molecular geometry or shape of the if 5 molecule is square pyramidal. A lone pair of electrons present on the central iodine atom in if. A=central atom, x= numbers of atoms bonded to. Xef 4 molecule is square planar in shape.
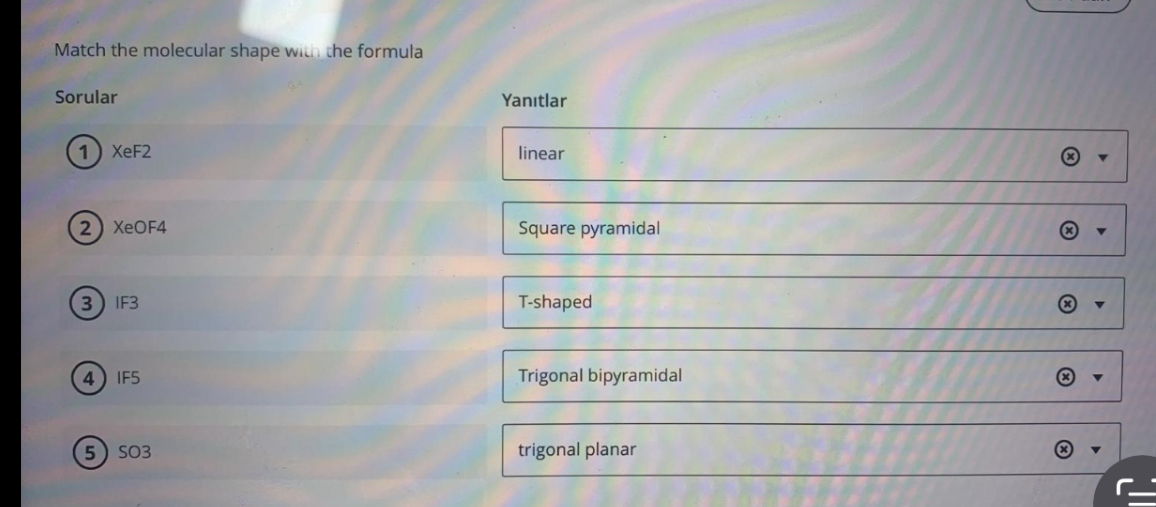
SOLVED Match the molecular shape with the formula Sorular (1) X e F 2
If 5 lewis structure shape. Xef 4 molecule is square planar in shape. The molecular geometry or shape of the if 5 molecule is square pyramidal. A lone pair of electrons present on the central iodine atom in if. Vsepr theory explained that if5 is ax 5 e type molecule.
If5 Lewis Structure Hybridization Polarity And Molecular Shape guidetech
Vsepr theory explained that if5 is ax 5 e type molecule. If 5 lewis structure shape. A=central atom, x= numbers of atoms bonded to. Xef 4 molecule is square planar in shape. The molecular geometry or shape of the if 5 molecule is square pyramidal.
SOLVED Text Draw the Lewis structure for the following compounds and
Vsepr theory explained that if5 is ax 5 e type molecule. A=central atom, x= numbers of atoms bonded to. The molecular geometry or shape of the if 5 molecule is square pyramidal. Xef 4 molecule is square planar in shape. If 5 lewis structure shape.
Xef 4 Molecule Is Square Planar In Shape.
If 5 lewis structure shape. Vsepr theory explained that if5 is ax 5 e type molecule. The molecular geometry or shape of the if 5 molecule is square pyramidal. A=central atom, x= numbers of atoms bonded to.