What Is The Molecular Geometry Of Clf4
What Is The Molecular Geometry Of Clf4 - Chlorine (cl) is the central atom, surrounded. An arrangement of four atoms or groups of atoms that form a. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion.
Chlorine (cl) is the central atom, surrounded. An arrangement of four atoms or groups of atoms that form a. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion.
To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a. Chlorine (cl) is the central atom, surrounded.
Clf4 Molecular Geometry
To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. Chlorine (cl) is the central atom, surrounded. An arrangement of four atoms or groups of atoms that form a.
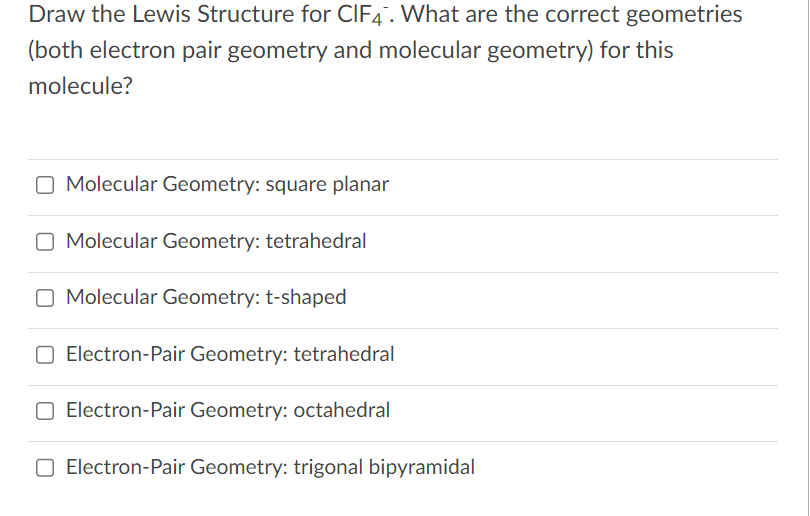
Solved Draw the Lewis Structure for ClF4. What are the
To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. Chlorine (cl) is the central atom, surrounded. An arrangement of four atoms or groups of atoms that form a.
Molecular Geometry of XeF4 [with video and free study guide]
Chlorine (cl) is the central atom, surrounded. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a.
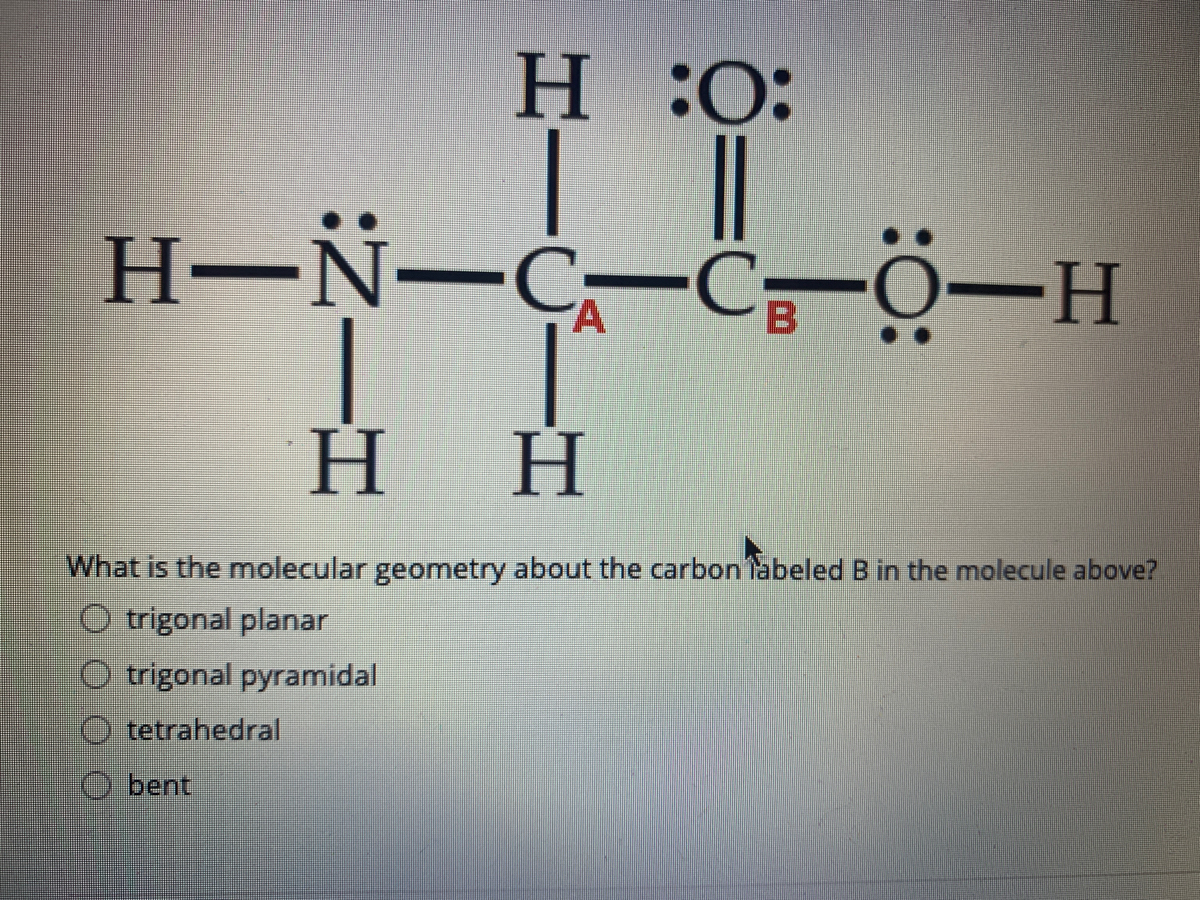
How To Draw Molecular Geometry
An arrangement of four atoms or groups of atoms that form a. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. Chlorine (cl) is the central atom, surrounded.
Electron and molecular geometry chart examples michaelhost
To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a. Chlorine (cl) is the central atom, surrounded.
Cf4 molecular geometry studentxoler
Chlorine (cl) is the central atom, surrounded. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a.
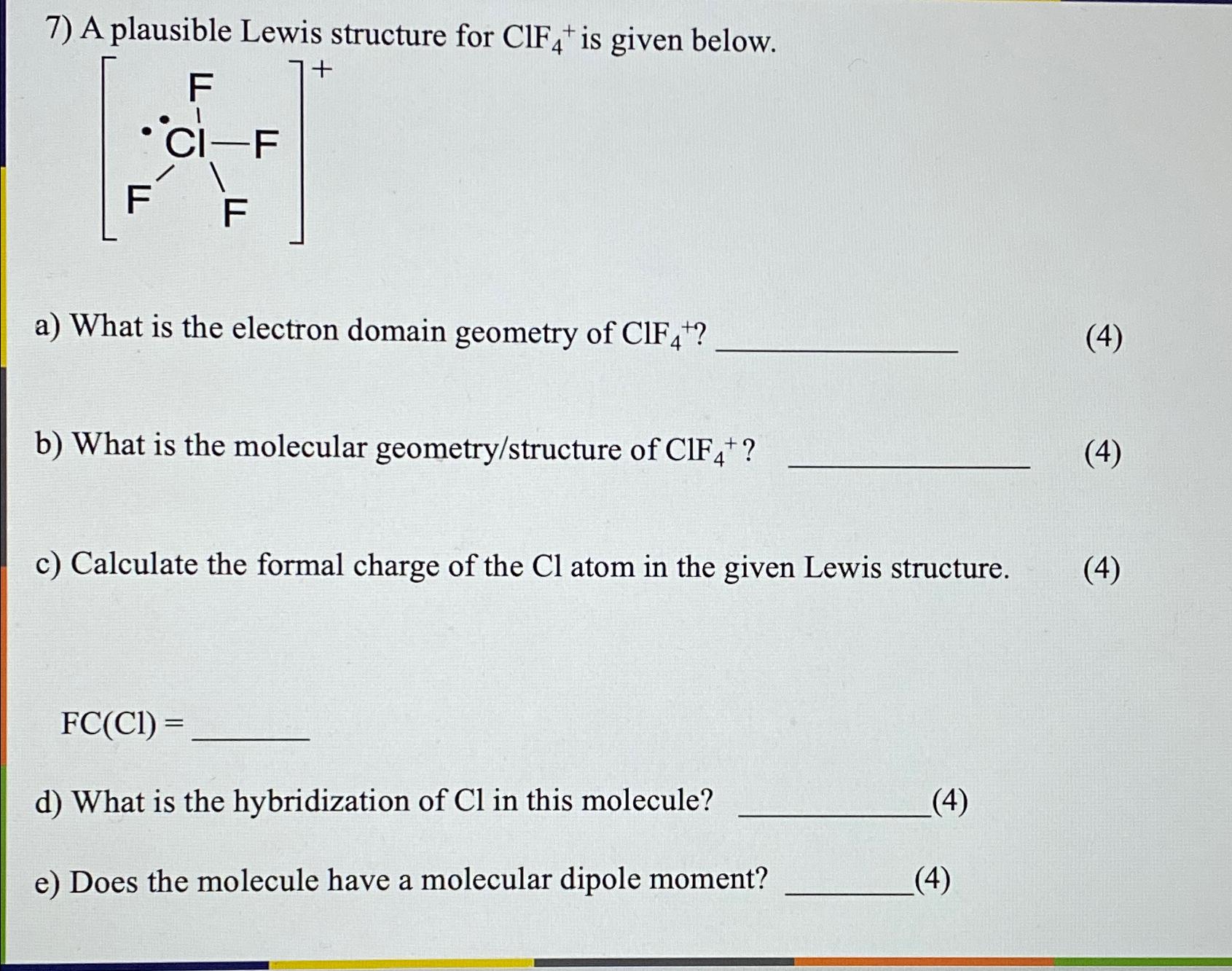
Solved A plausible Lewis structure for ClF4+is given
Chlorine (cl) is the central atom, surrounded. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a.
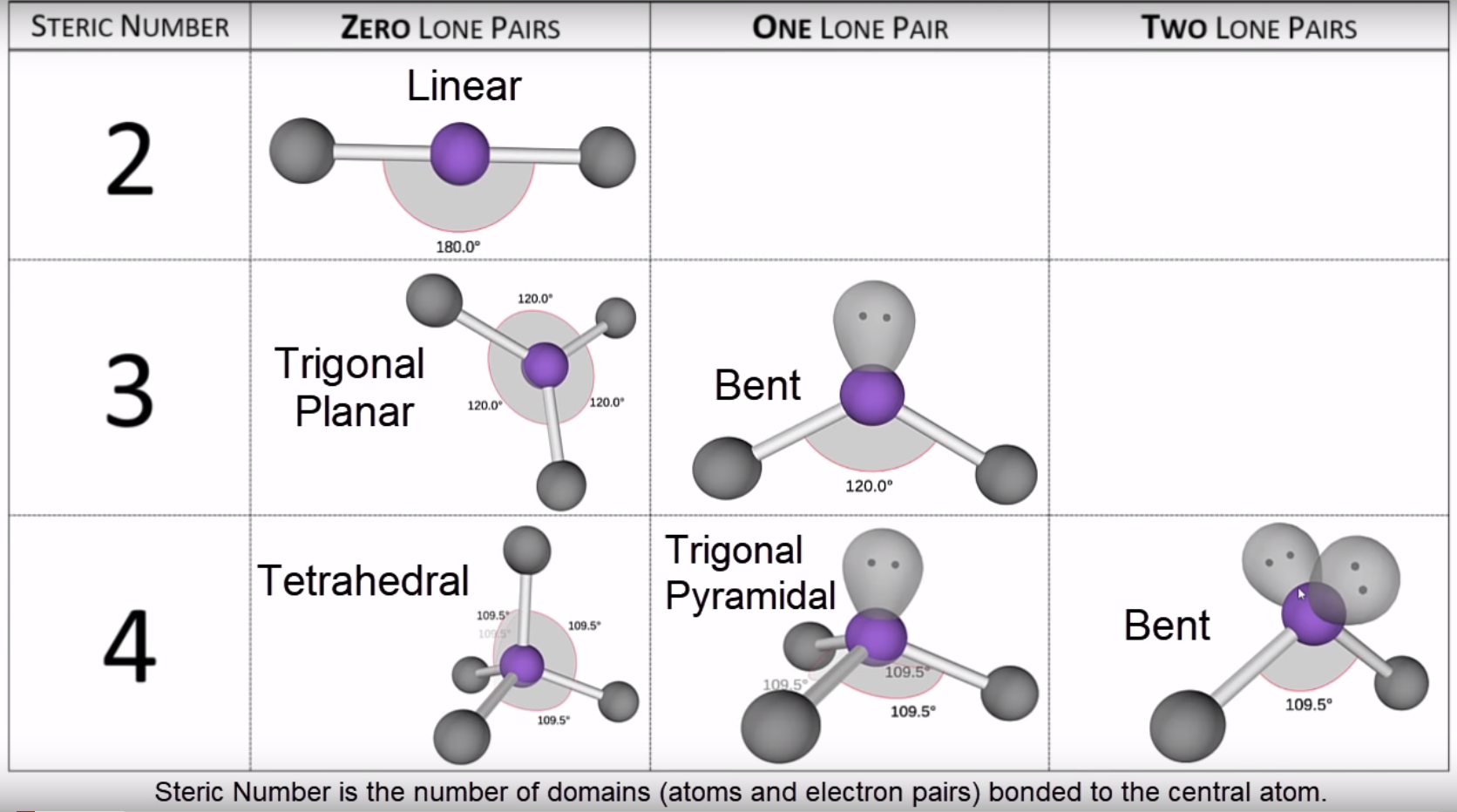
Molecular Geometry Chart
To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. An arrangement of four atoms or groups of atoms that form a. Chlorine (cl) is the central atom, surrounded.
Clf4 Molecular Geometry
An arrangement of four atoms or groups of atoms that form a. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion. Chlorine (cl) is the central atom, surrounded.
Chlorine (Cl) Is The Central Atom, Surrounded.
An arrangement of four atoms or groups of atoms that form a. To determine the molecular geometry of the clf4− ion, we start by sketching the lewis structure for the ion.

![Molecular Geometry of XeF4 [with video and free study guide]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/VSEPR-geomtries-chart.jpg)