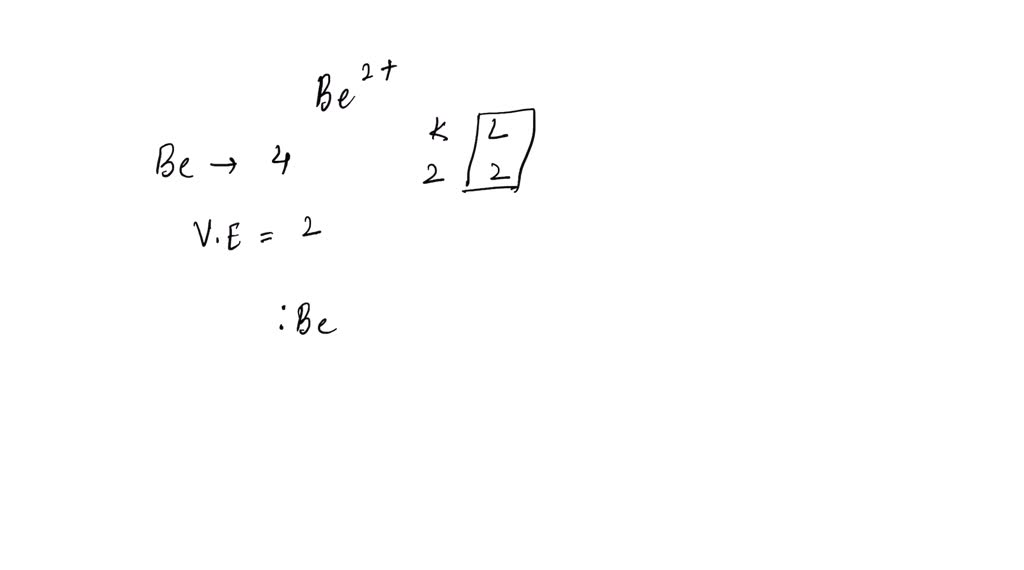What Is The Lewis Symbol For Be2
What Is The Lewis Symbol For Be2 - What is the lewis symbol for be2+? In the case of be2+ (beryllium. What is the lewis symbol for k+? The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. When drawing the lewis structure for a molecule, after drawing the.
A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. In the case of be2+ (beryllium. What is the lewis symbol for be2+? The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. What is the lewis symbol for k+? When drawing the lewis structure for a molecule, after drawing the.
When drawing the lewis structure for a molecule, after drawing the. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. What is the lewis symbol for k+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. What is the lewis symbol for be2+? In the case of be2+ (beryllium.
What Is The Lewis Symbol For Be2+ symbol
What is the lewis symbol for k+? The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. What is the.
SOLVED What is the Lewis symbol for Be Select the correct answer below
When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? In the case of be2+ (beryllium. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2.
What Is The Lewis Symbol For Be2+ symbol
In the case of be2+ (beryllium. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. What is the lewis symbol for be2+? What is the lewis symbol for k+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with.
BeI2 Lewis Structure, Geometry, Hybridization, and Polarity
A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive.
What Is The Lewis Symbol For Be2+ symbol
When drawing the lewis structure for a molecule, after drawing the. The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. What is the lewis symbol for be2+? The.
SOLVED The Lewis symbol for Ba2+ is Ba2+
When drawing the lewis structure for a molecule, after drawing the. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. What is the lewis symbol for be2+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. What.
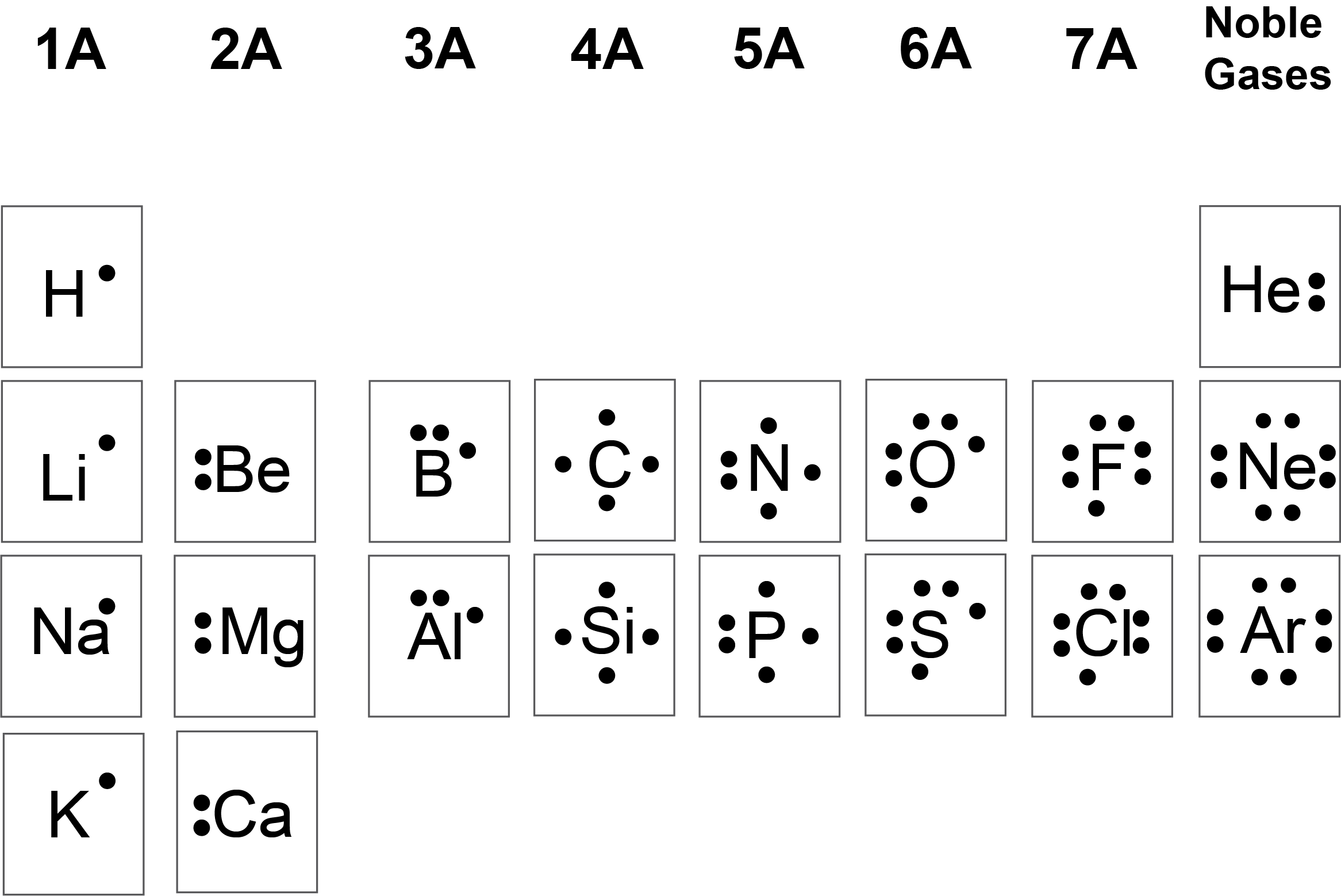
15 Lewis Dot Structure Examples Robhosking Diagram
A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. What is the lewis symbol for k+? When drawing the lewis structure for a molecule, after drawing the. What is the lewis symbol for be2+? In the case of be2+ (beryllium.
Bebr2 Lewis Structure, Characteristics 13 Must To Know Facts Lambda
The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. In the case of be2+ (beryllium. A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. When drawing the lewis structure for a molecule, after drawing the. The lewis.
28+ Lewis Diagram Hcn AdenJarlath
The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. A basic lewis symbol consists of an elemental symbol surrounded.
What Is The Lewis Symbol For Be2+ symbol
What is the lewis symbol for be2+? The lewis structure of the beryllium ion, be2+, shows a beryllium atom with two positive charges, indicating the loss of its two valence. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence. A basic lewis.
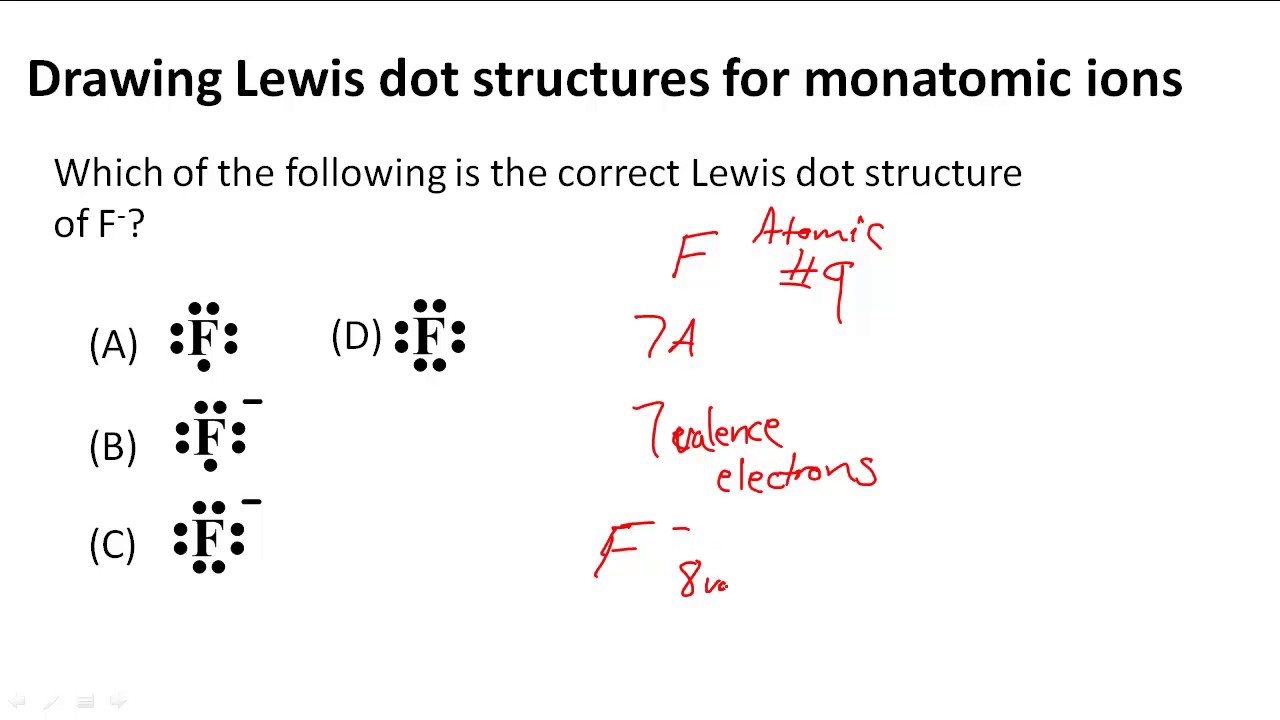
What Is The Lewis Symbol For K+?
A basic lewis symbol consists of an elemental symbol surrounded by dots for each of its valence electrons. What is the lewis symbol for be2+? When drawing the lewis structure for a molecule, after drawing the. The lewis symbol for a be2+ ion is a beryllium atom with zero valence electrons and a +2 charge, indicating that it has lost its two valence.
The Lewis Structure Of The Beryllium Ion, Be2+, Shows A Beryllium Atom With Two Positive Charges, Indicating The Loss Of Its Two Valence.
In the case of be2+ (beryllium.