What Is The Hybridization Of The Nitrogen Atoms In N2
What Is The Hybridization Of The Nitrogen Atoms In N2 - Nitrogen gas is shown below. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. It seems like hybridization of atomic orbitals only occur for central atoms and not for. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. It has a triple bond and one lone pair on each nitrogen.
Nitrogen gas is shown below. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. It has a triple bond and one lone pair on each nitrogen. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. It seems like hybridization of atomic orbitals only occur for central atoms and not for. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is.
It has a triple bond and one lone pair on each nitrogen. It seems like hybridization of atomic orbitals only occur for central atoms and not for. Nitrogen gas is shown below. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,.
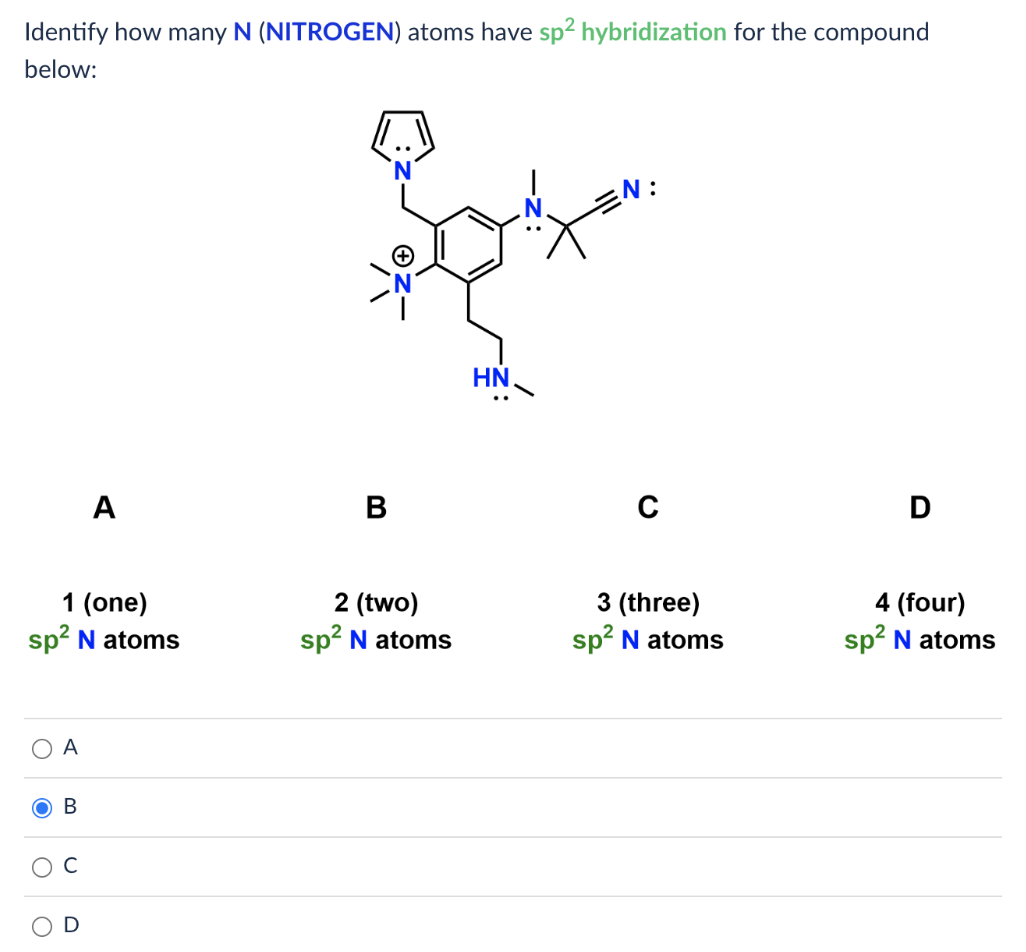
[Solved] Identify how many N (NITROGEN) atoms have sp hybridization for
I was just going over the 2011 midterm and am having a lot of trouble with question 6c. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. It has a triple bond and one lone pair on each nitrogen. It seems like.
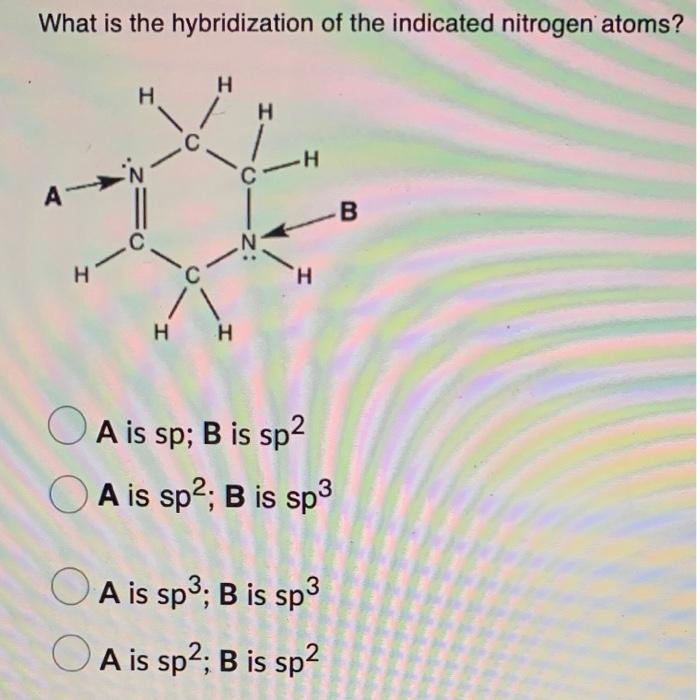
Solved What is the hybridization of the indicated nitrogen
I was just going over the 2011 midterm and am having a lot of trouble with question 6c. It has a triple bond and one lone pair on each nitrogen. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. Nitrogen gas is shown below. You may want to memorize the diatomic elements (hydrogen,.
[Solved] Identify how many N (NITROGEN) atoms have sp2
Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. It seems like hybridization of atomic orbitals only occur for central atoms and not for..
Solved Identify how many N (NITROGEN) atoms have \\(
It has a triple bond and one lone pair on each nitrogen. It seems like hybridization of atomic orbitals only occur for central atoms and not for. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. Nitrogen gas is shown.
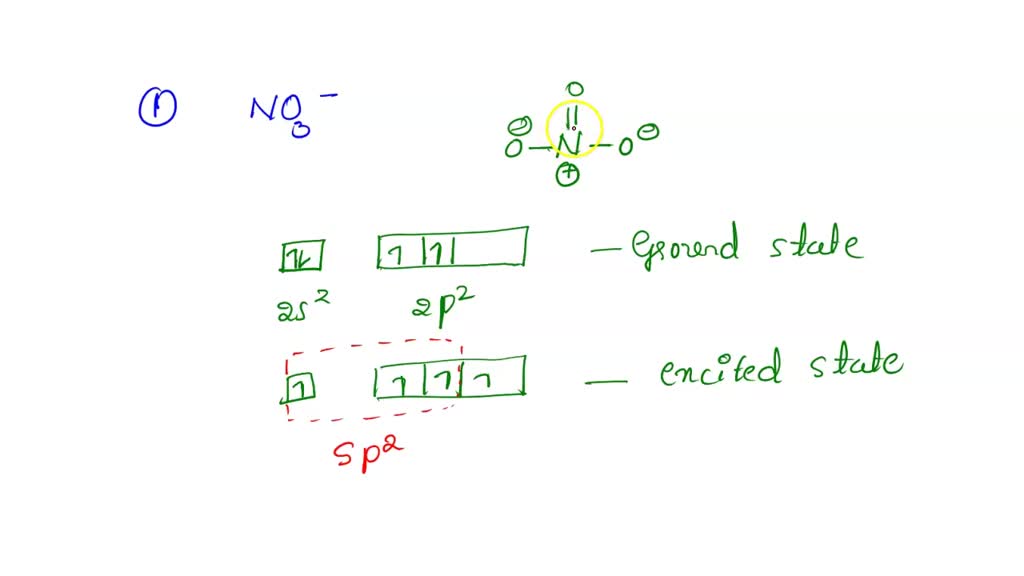
SOLVED What is the hybridization of nitrogen in the nitrite ion
It has a triple bond and one lone pair on each nitrogen. It seems like hybridization of atomic orbitals only occur for central atoms and not for. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. Since it is talking.
Solved What is the hybridization of the indicated nitrogen
Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. Nitrogen gas is shown below. It seems like hybridization of atomic orbitals only occur for central atoms and not for. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. It has a triple bond and one lone pair on each nitrogen.
[Solved] Identify how many N (NITROGEN) atoms have sp hybridization for
Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. It has a triple bond and one lone pair on each nitrogen. It seems like hybridization of atomic orbitals only occur for central atoms and not for. I was just going over the 2011 midterm and am having a lot of trouble with question.
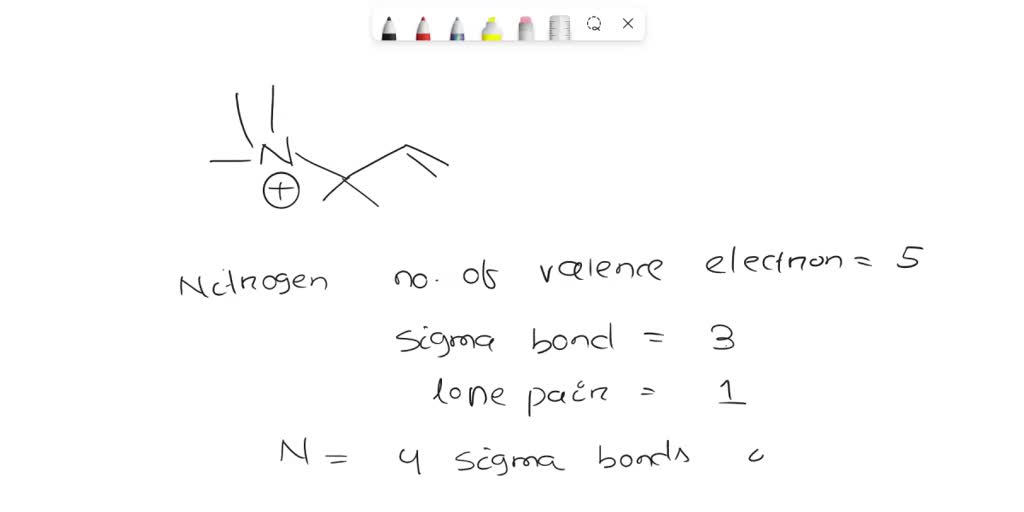
SOLVED Please identify the type of hybridization (sp, sp2, sp3) for
Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. Nitrogen gas is shown below. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. It has a triple bond and one lone pair on each nitrogen. You may want to memorize the diatomic elements (hydrogen,.
[Solved] Identify how many N ( NITROGEN ) atoms have sp 3 hybridization
Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. It seems like hybridization of atomic orbitals only occur for central atoms and not for..
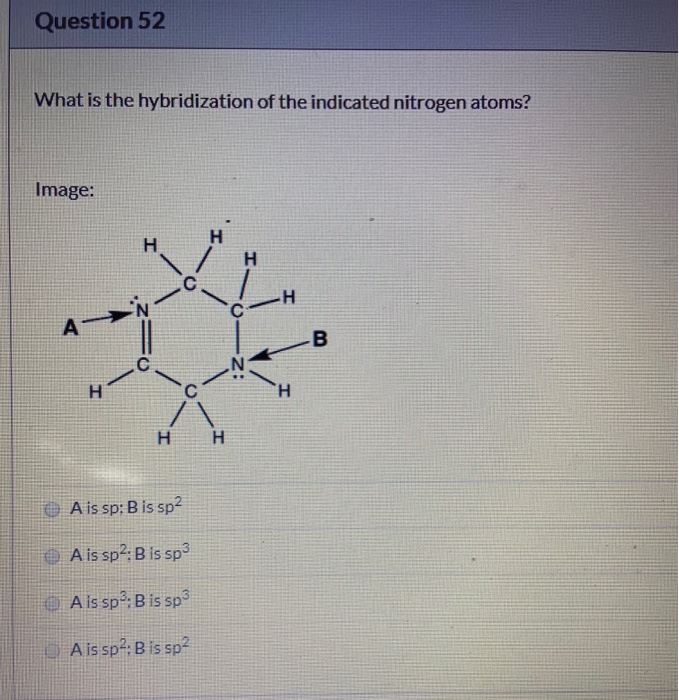
Solved Question 52 What Is The Hybridization Of The Indic...
Nitrogen gas is shown below. Since it is talking about a nitrogen atmosphere, i think it means that the nitrogen is. It seems like hybridization of atomic orbitals only occur for central atoms and not for. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. I was just going over the 2011 midterm and am having a lot.
Since It Is Talking About A Nitrogen Atmosphere, I Think It Means That The Nitrogen Is.
It seems like hybridization of atomic orbitals only occur for central atoms and not for. You may want to memorize the diatomic elements (hydrogen, nitrogen, fluorine,. I was just going over the 2011 midterm and am having a lot of trouble with question 6c. Nitrogen gas is shown below.
![[Solved] Identify how many N (NITROGEN) atoms have sp2](https://media.cheggcdn.com/media/250/25001471-53e2-40e1-97ef-668977575fc1/phppbkvdZ)