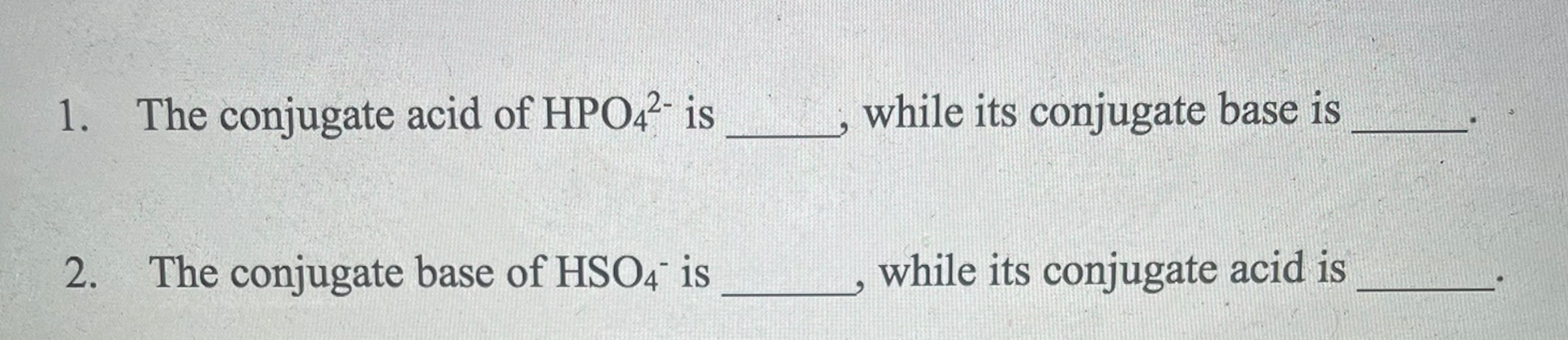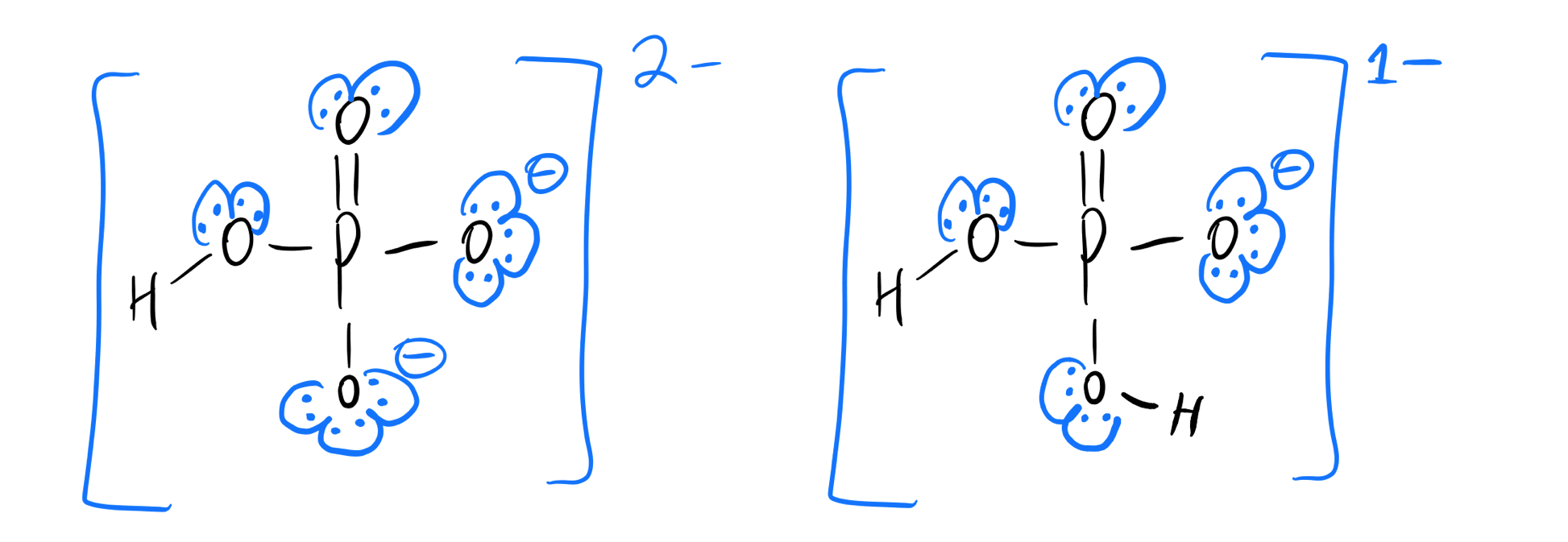What Is The Conjugate Acid Of Hpo42
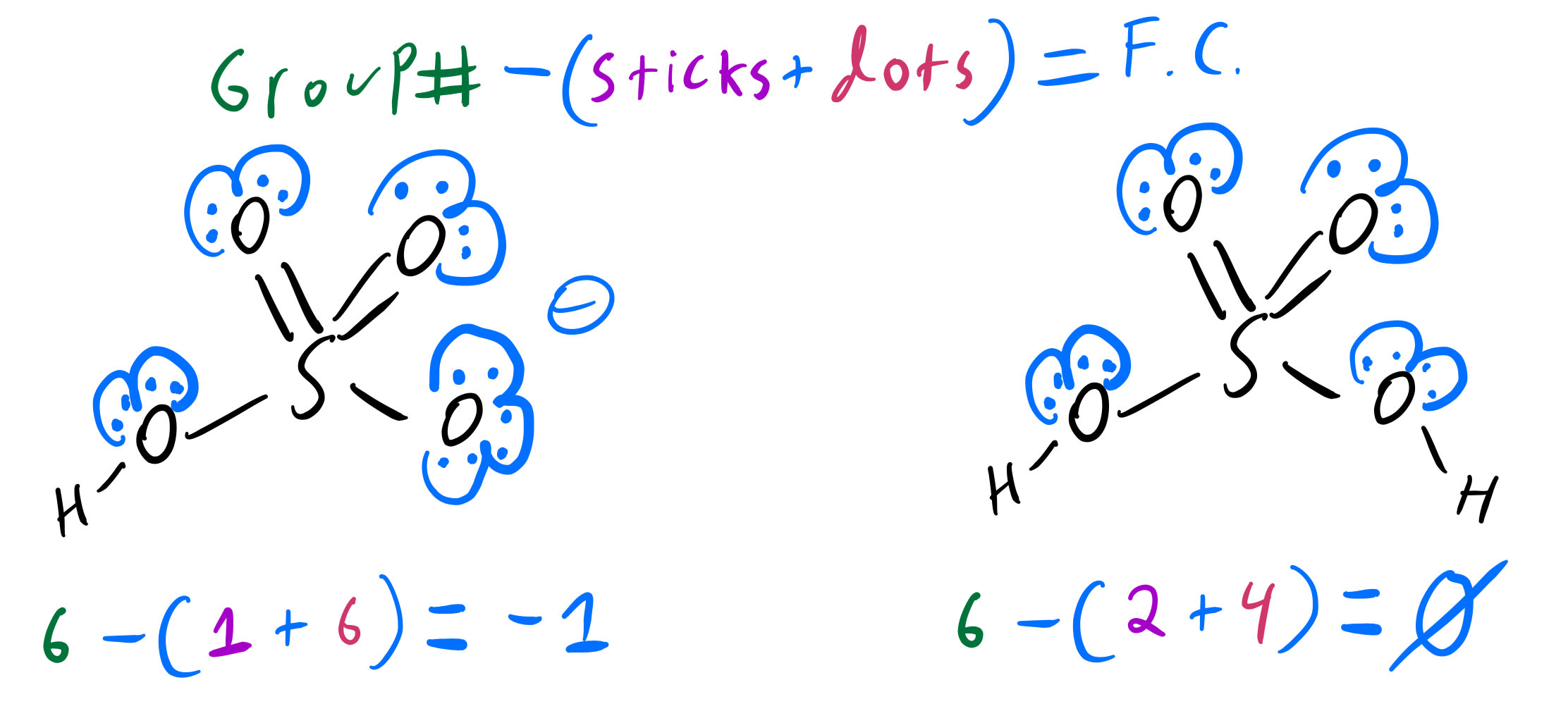
What Is The Conjugate Acid Of Hpo42 - The question asks for the conjugate. The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. Consider various species generated when h 3p o4 is dissolved in water. According to the bronsted lowry’s concept among these, the conjugate acid. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: This is because a conjugate acid is formed when a base gains a proton (h+).
This is because a conjugate acid is formed when a base gains a proton (h+). The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: The question asks for the conjugate. Consider various species generated when h 3p o4 is dissolved in water. According to the bronsted lowry’s concept among these, the conjugate acid.
The question asks for the conjugate. This is because a conjugate acid is formed when a base gains a proton (h+). According to the bronsted lowry’s concept among these, the conjugate acid. The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: Consider various species generated when h 3p o4 is dissolved in water.
Conjugate Acid Of Hpo42 Asking List
The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. Consider various species generated when h 3p o4 is dissolved in water. According to the bronsted lowry’s concept among these, the conjugate acid. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺.
SOLVED The pKas of the conjugate acids of OH (conjugate acid H2O) and
According to the bronsted lowry’s concept among these, the conjugate acid. The question asks for the conjugate. Consider various species generated when h 3p o4 is dissolved in water. The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. This is because a conjugate acid is formed when a base.
Conjugate Acid Of H2po4 Asking List
This is because a conjugate acid is formed when a base gains a proton (h+). The question asks for the conjugate. Consider various species generated when h 3p o4 is dissolved in water. According to the bronsted lowry’s concept among these, the conjugate acid. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d).
Solved What are the conjugate base and conjugate acid of
The question asks for the conjugate. The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. This is because a conjugate acid is formed when a base gains a proton (h+). Consider various species generated when h 3p o4 is dissolved in water. According to the bronsted lowry’s concept among.
Conjugate Acid Of H2po4 Asking List
The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. According to the bronsted lowry’s concept among these, the conjugate acid. Consider various species generated when h 3p o4 is dissolved in water. This is because a conjugate acid is formed when a base gains a proton (h+). The question.
Solved 1. The conjugate acid of HPO42− is , while its
Consider various species generated when h 3p o4 is dissolved in water. The question asks for the conjugate. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: According to the bronsted lowry’s concept among these, the conjugate acid. The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only.
Give the conjugate acid for each compound below Organic Chemistry
The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only proton that separates the base from its. This is because a conjugate acid is formed when a base gains a proton (h+). The question asks for the conjugate. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: Consider.
SOLVED Fill in the missing chemical formulae in the tables below acid
The question asks for the conjugate. This is because a conjugate acid is formed when a base gains a proton (h+). The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: According to the bronsted lowry’s concept among these, the conjugate acid. Consider various species generated when h 3p o4 is dissolved.
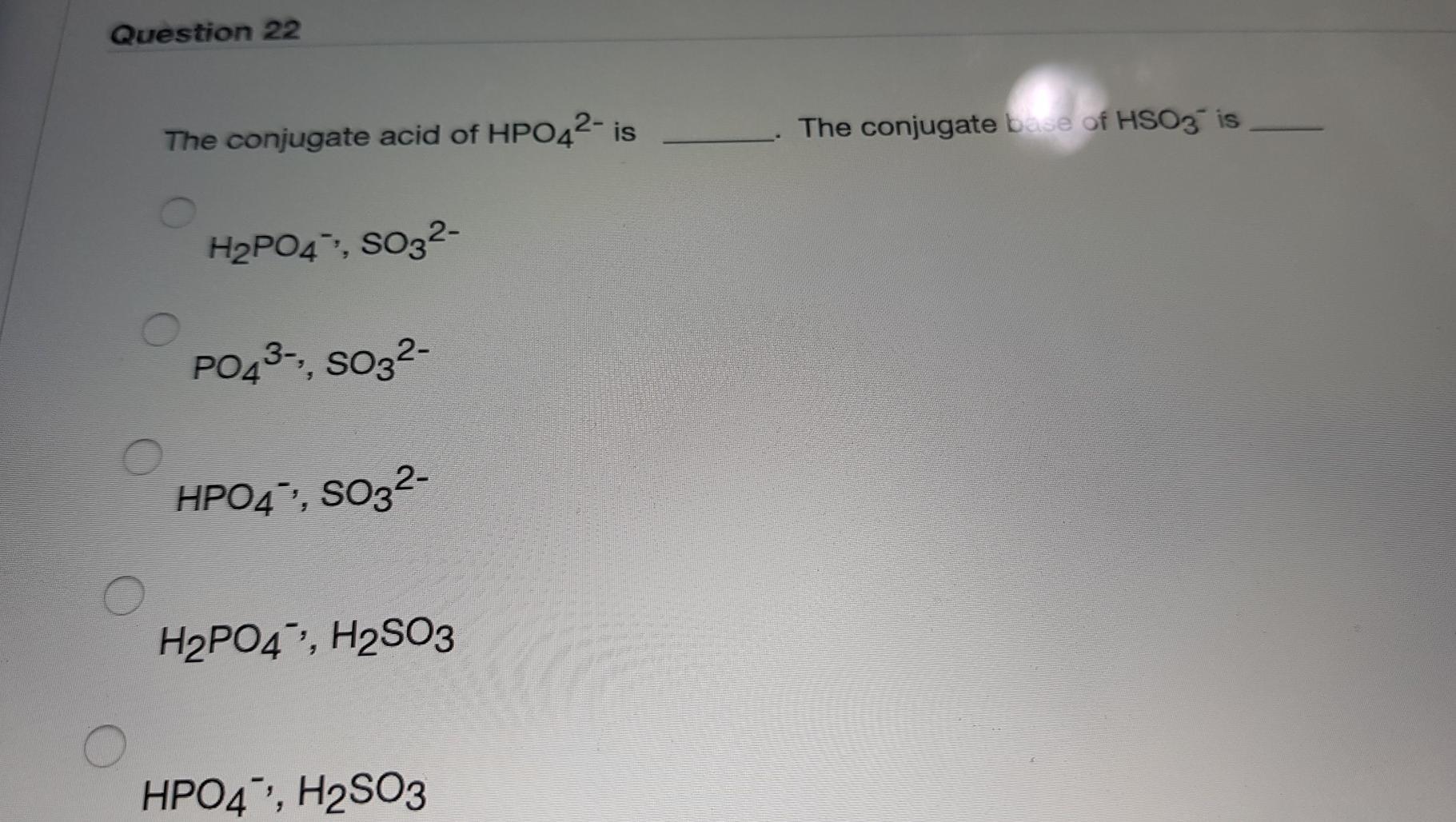
Solved Question 22 The conjugate acid of HPO42 is The
The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: This is because a conjugate acid is formed when a base gains a proton (h+). The question asks for the conjugate. According to the bronsted lowry’s concept among these, the conjugate acid. The correct answer is conjugate acid of hpo42− = h2po4−the.
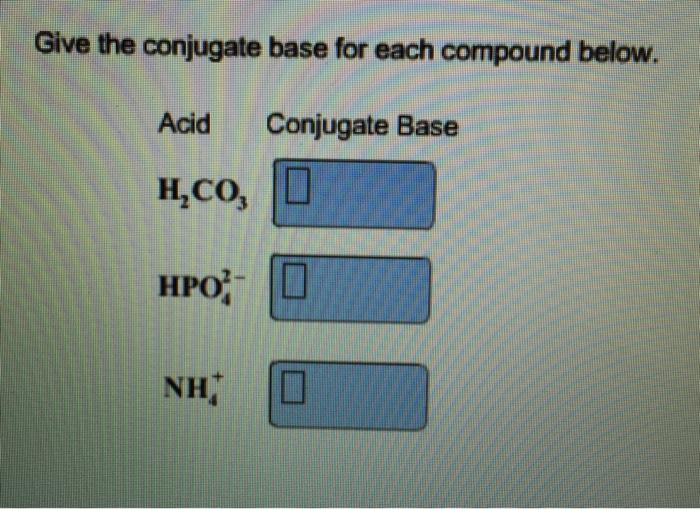
Solved Give the conjugate base for each compound below. Acid
Consider various species generated when h 3p o4 is dissolved in water. The question asks for the conjugate. According to the bronsted lowry’s concept among these, the conjugate acid. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: The correct answer is conjugate acid of hpo42− = h2po4−the reaction is,h2po4−→h++hpo42−the only.
According To The Bronsted Lowry’s Concept Among These, The Conjugate Acid.
The question asks for the conjugate. The conjugate acid of hpo₄²⁻ is h₂po₄⁻ which is formed when hpo₄²⁻ gains a proton d) h⁺ explanation: This is because a conjugate acid is formed when a base gains a proton (h+). Consider various species generated when h 3p o4 is dissolved in water.