What Is The Bond Order For O2
What Is The Bond Order For O2 - The bond order of a covalent bond is the total number of covalently bonded electron pairs. The bond order shows the number of chemical bonds present between a pair of atoms. Each molecule has a different bond arrangement;. Every molecule has a bonding hierarchy. “in the lewis description of covalent bond, the bond order is given by the number of.
The bond order shows the number of chemical bonds present between a pair of atoms. The bond order of a covalent bond is the total number of covalently bonded electron pairs. Each molecule has a different bond arrangement;. “in the lewis description of covalent bond, the bond order is given by the number of. Every molecule has a bonding hierarchy.
The bond order of a covalent bond is the total number of covalently bonded electron pairs. The bond order shows the number of chemical bonds present between a pair of atoms. “in the lewis description of covalent bond, the bond order is given by the number of. Each molecule has a different bond arrangement;. Every molecule has a bonding hierarchy.
What is meant by term bond order? Write bond orders for N2, O2?
Every molecule has a bonding hierarchy. Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. “in the lewis description of covalent bond, the bond order is given by the number of. The bond order shows the number of chemical bonds present between a pair of atoms.
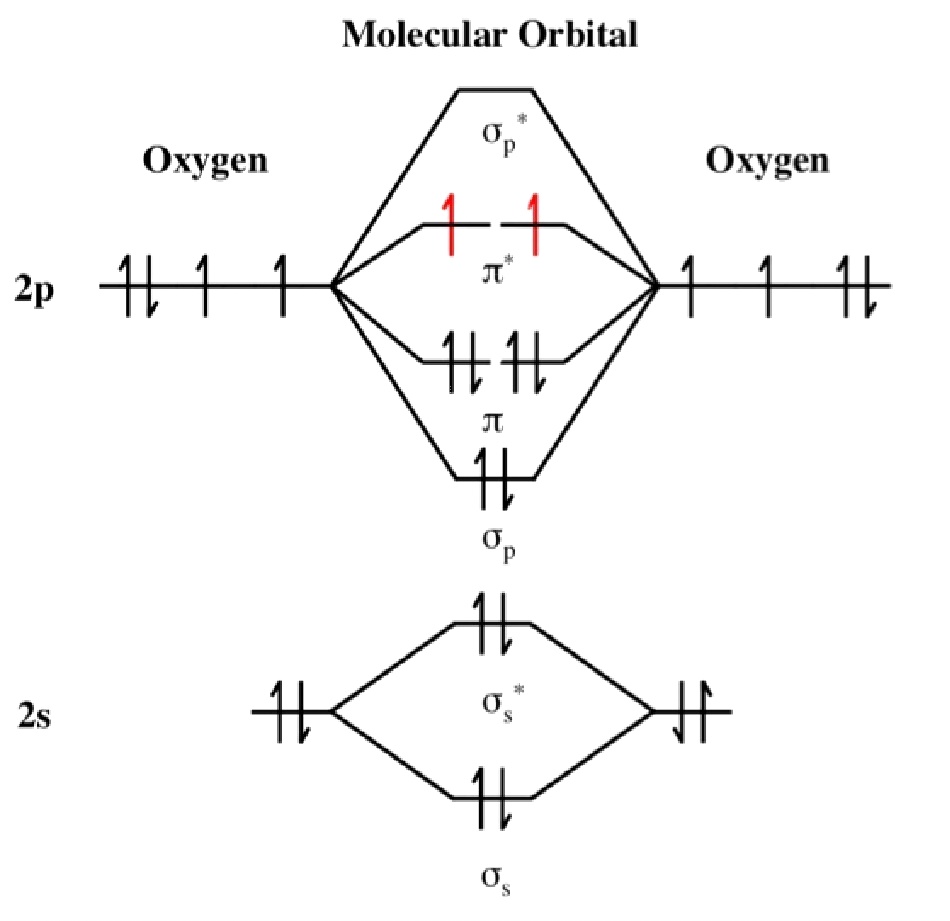
MO DIAGRAM of O2,O2,O2+ THEIR BOND ORDER AND CHAR
Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. The bond order shows the number of chemical bonds present between a pair of atoms. “in the lewis description of covalent bond, the bond order is given by the number of. Every molecule has a bonding hierarchy.
Explain why the bond order of N2 is greater than N2+, but the bond
The bond order shows the number of chemical bonds present between a pair of atoms. Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. Every molecule has a bonding hierarchy. “in the lewis description of covalent bond, the bond order is given by the number of.
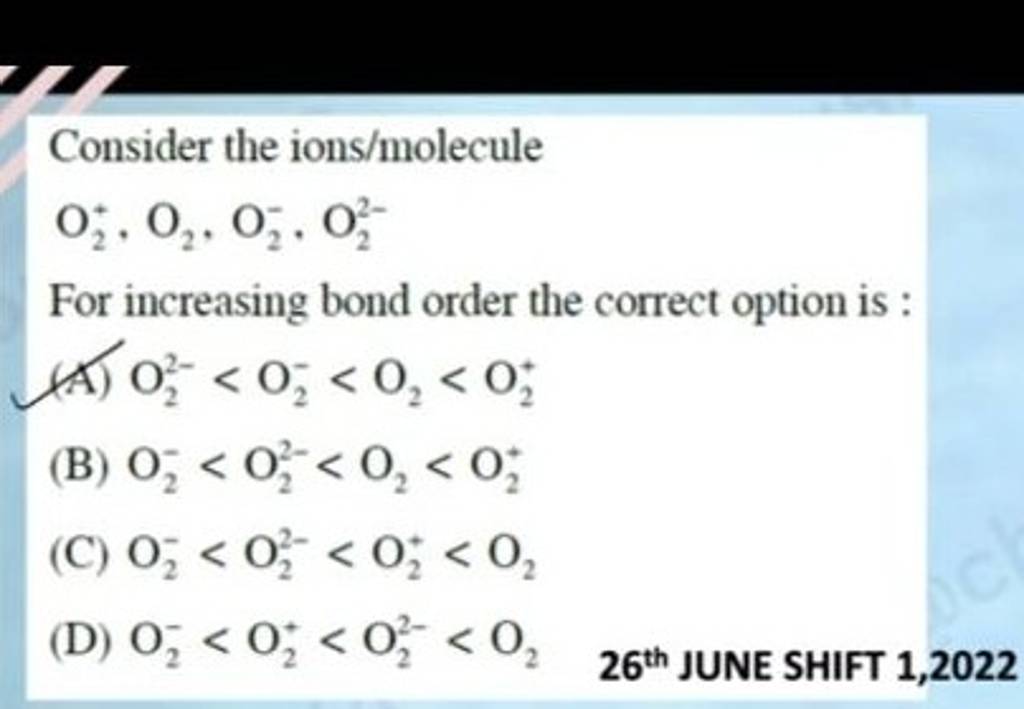
Consider the ions/molecule O2+ ,O2 ,O2− ,O22− For increasing bond order
Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. The bond order shows the number of chemical bonds present between a pair of atoms. Every molecule has a bonding hierarchy. “in the lewis description of covalent bond, the bond order is given by the number of.
Calculate the bond order in O_2,O_2^,O_2^{2} and O_2^+ molecule.
The bond order shows the number of chemical bonds present between a pair of atoms. Each molecule has a different bond arrangement;. Every molecule has a bonding hierarchy. “in the lewis description of covalent bond, the bond order is given by the number of. The bond order of a covalent bond is the total number of covalently bonded electron pairs.
The bond order of O_2^+ is the same as in
Each molecule has a different bond arrangement;. The bond order shows the number of chemical bonds present between a pair of atoms. “in the lewis description of covalent bond, the bond order is given by the number of. Every molecule has a bonding hierarchy. The bond order of a covalent bond is the total number of covalently bonded electron pairs.
Bond Order Of N2 astonishingceiyrs
The bond order shows the number of chemical bonds present between a pair of atoms. Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. Every molecule has a bonding hierarchy. “in the lewis description of covalent bond, the bond order is given by the number of.
Calculate the bond order of N2,O2,O2^+and O2^ Chemistry Chemical
The bond order shows the number of chemical bonds present between a pair of atoms. Every molecule has a bonding hierarchy. The bond order of a covalent bond is the total number of covalently bonded electron pairs. “in the lewis description of covalent bond, the bond order is given by the number of. Each molecule has a different bond arrangement;.
How To Calculate Bond Order
The bond order shows the number of chemical bonds present between a pair of atoms. The bond order of a covalent bond is the total number of covalently bonded electron pairs. “in the lewis description of covalent bond, the bond order is given by the number of. Every molecule has a bonding hierarchy. Each molecule has a different bond arrangement;.
O2 Bond Order Diagram
Every molecule has a bonding hierarchy. The bond order shows the number of chemical bonds present between a pair of atoms. The bond order of a covalent bond is the total number of covalently bonded electron pairs. “in the lewis description of covalent bond, the bond order is given by the number of. Each molecule has a different bond arrangement;.
“In The Lewis Description Of Covalent Bond, The Bond Order Is Given By The Number Of.
Each molecule has a different bond arrangement;. The bond order of a covalent bond is the total number of covalently bonded electron pairs. Every molecule has a bonding hierarchy. The bond order shows the number of chemical bonds present between a pair of atoms.