What Is The Bond Order For No2
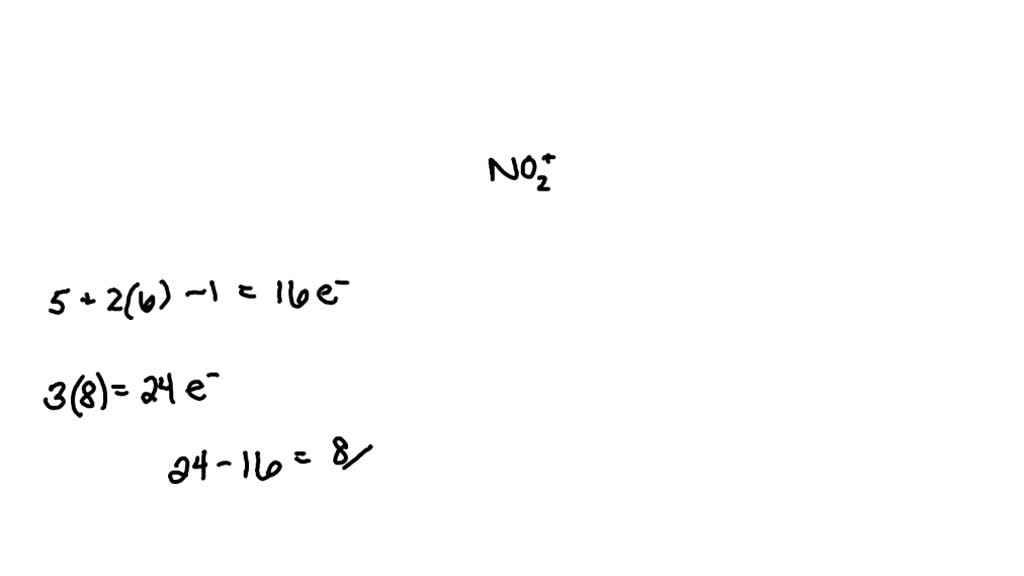
What Is The Bond Order For No2 - Its bond order is 2. Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for no2 can be calculated as follows: If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by:
The bond order for the given molecule is given by: The bond order for no2 can be calculated as follows: Thus, no^(2+) loses the 2b_1 antibonding electron and. Its bond order is 2. If you mean no^(2+), the mo diagram of no is:
If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by: Its bond order is 2. The bond order for no2 can be calculated as follows: Thus, no^(2+) loses the 2b_1 antibonding electron and.
The correct order for N O bond length in the given species isI. NO
Its bond order is 2. The bond order for no2 can be calculated as follows: Thus, no^(2+) loses the 2b_1 antibonding electron and. If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by:
[Solved] Which one has a higher bond order, NO2 or NO3 ? Show your
Its bond order is 2. The bond order for no2 can be calculated as follows: If you mean no^(2+), the mo diagram of no is: Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for the given molecule is given by:
Among NO2 ,NO2+,NO3 , what is the order of bond length and bond angle
Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for the given molecule is given by: The bond order for no2 can be calculated as follows: If you mean no^(2+), the mo diagram of no is: Its bond order is 2.
How To Calculate Bond Order
The bond order for no2 can be calculated as follows: Its bond order is 2. Thus, no^(2+) loses the 2b_1 antibonding electron and. If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by:
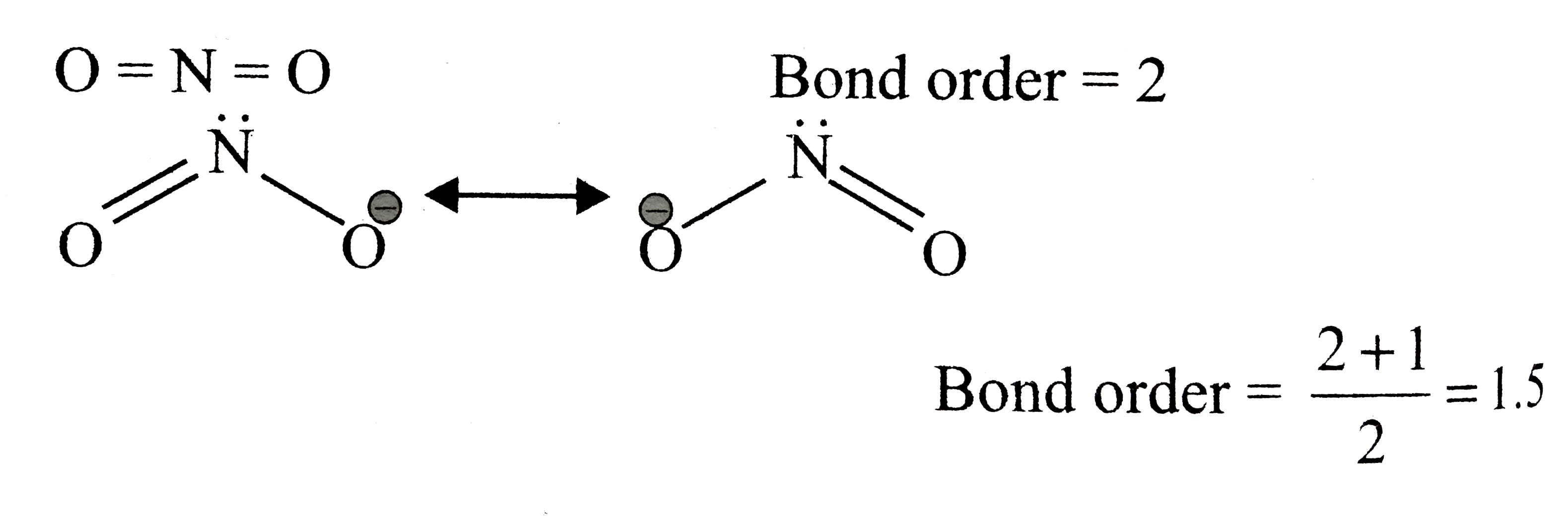
Solved The NO bond order in [NO2]−is best described as 1 121
Its bond order is 2. The bond order for no2 can be calculated as follows: Thus, no^(2+) loses the 2b_1 antibonding electron and. If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by:
How to find Bond Order of (NO2)+ Chemistry 13524081
Thus, no^(2+) loses the 2b_1 antibonding electron and. Its bond order is 2. If you mean no^(2+), the mo diagram of no is: The bond order for no2 can be calculated as follows: The bond order for the given molecule is given by:
Order of bond angle among NO2 ,NO2+ ,NO2− is Filo
Its bond order is 2. If you mean no^(2+), the mo diagram of no is: The bond order for no2 can be calculated as follows: The bond order for the given molecule is given by: Thus, no^(2+) loses the 2b_1 antibonding electron and.
What is the bond order in NO2+?
If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by: Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for no2 can be calculated as follows: Its bond order is 2.
SOLVED calculate the bond order for NO2+ ion, regarding all the
Its bond order is 2. If you mean no^(2+), the mo diagram of no is: The bond order for the given molecule is given by: Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for no2 can be calculated as follows:
26. The correct decreasing order of bond angles of NO2− ,NO2+ and NO2 is..
If you mean no^(2+), the mo diagram of no is: Its bond order is 2. The bond order for no2 can be calculated as follows: Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for the given molecule is given by:
If You Mean No^(2+), The Mo Diagram Of No Is:
The bond order for the given molecule is given by: Its bond order is 2. Thus, no^(2+) loses the 2b_1 antibonding electron and. The bond order for no2 can be calculated as follows:

![Solved The NO bond order in [NO2]−is best described as 1 121](https://media.cheggcdn.com/media/2d4/2d47a7dd-9309-4816-ab40-22e1add0f061/php53XYm4)