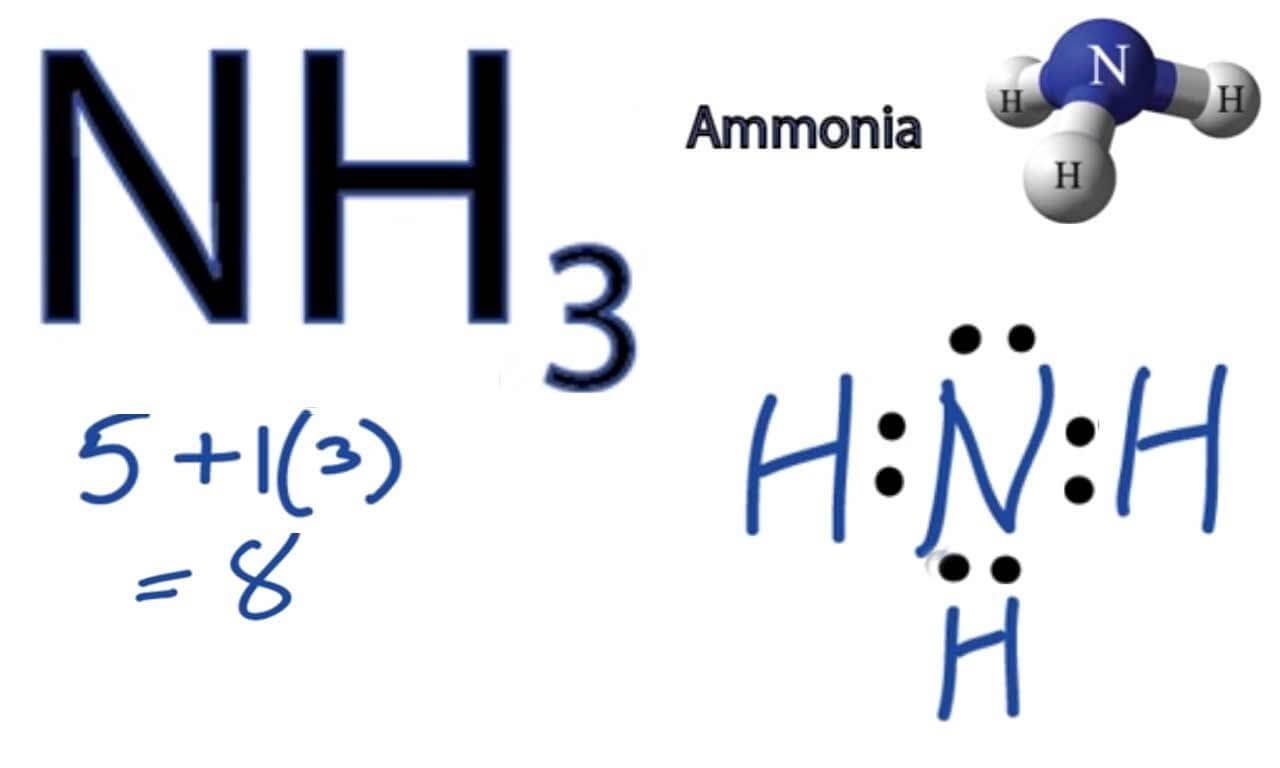What Is The Bond Angle Of Nh3
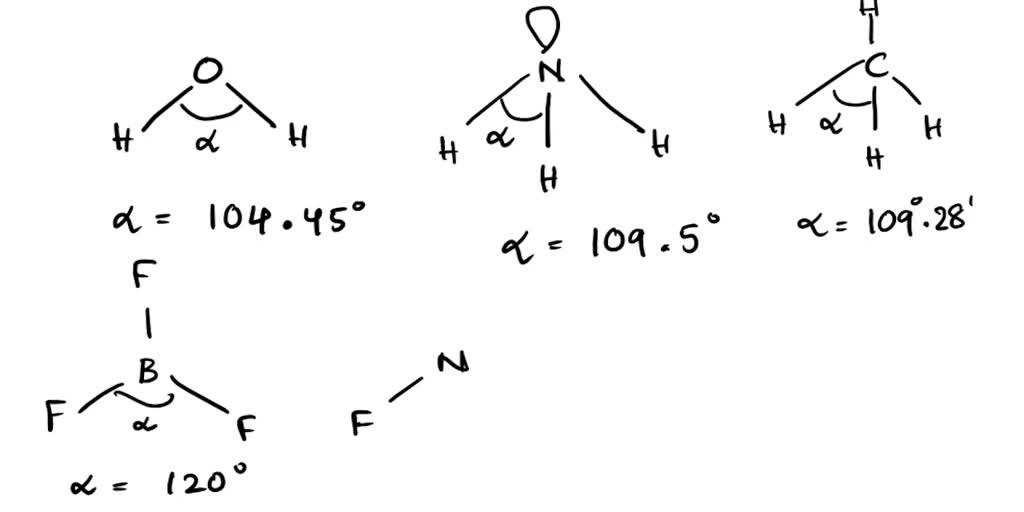
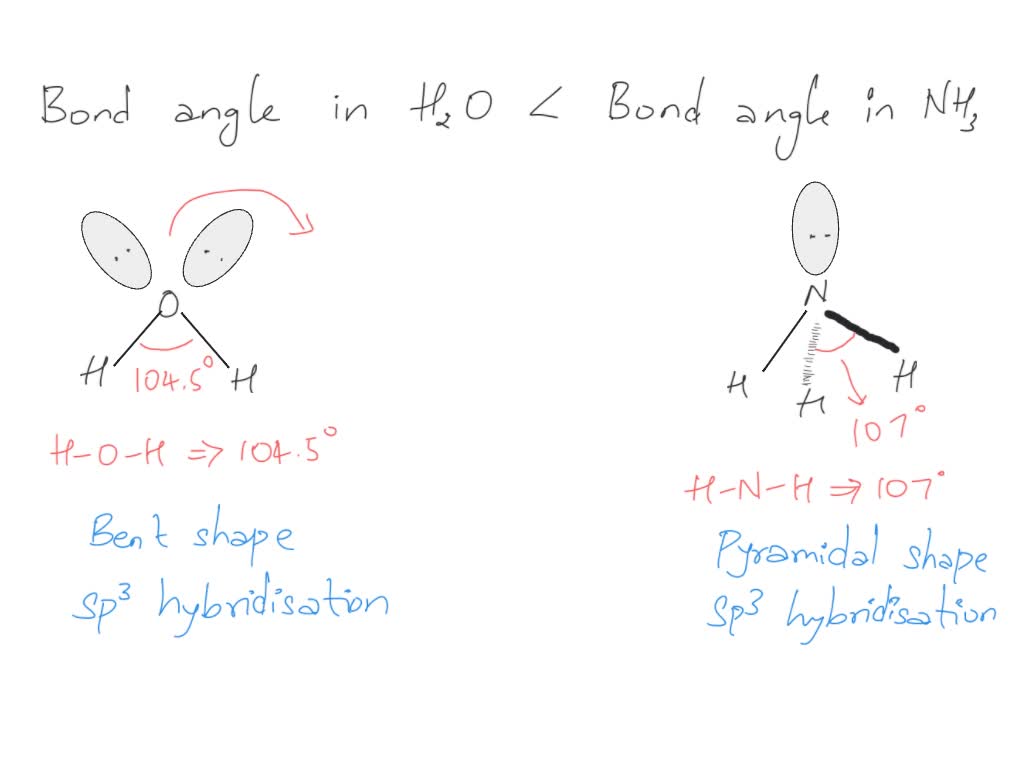
What Is The Bond Angle Of Nh3 - As we move down the group, radius of elements increases and electronegativity decreases so. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. Hence repulsion between bond pairs in n f 3, is less than n h 3. As water has two lone pairs the. As a result the bond angle decreases.
Hence repulsion between bond pairs in n f 3, is less than n h 3. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As a result the bond angle decreases. As we move down the group, radius of elements increases and electronegativity decreases so. As water has two lone pairs the.
So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. Hence repulsion between bond pairs in n f 3, is less than n h 3. As water has two lone pairs the. As a result the bond angle decreases. As we move down the group, radius of elements increases and electronegativity decreases so.
Explain why the bond angle of NH3 is greater than that of NF3 while the
As water has two lone pairs the. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As we move down the group, radius of elements increases and electronegativity decreases so. Hence repulsion between bond pairs in n f 3, is less than n h 3. As a result the bond.
Correct order of bond angle in the given molecules (1) H2 O>NH3 >CH4
As we move down the group, radius of elements increases and electronegativity decreases so. As water has two lone pairs the. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. Hence repulsion between bond pairs in n f 3, is less than n h 3. As a result the bond.
Explain why the bond angle of NH3 is greater than that of NF3 while the
Hence repulsion between bond pairs in n f 3, is less than n h 3. As we move down the group, radius of elements increases and electronegativity decreases so. As water has two lone pairs the. As a result the bond angle decreases. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about.
SOLVED Compare the bond angle predicted from VSEPR Theory and the bond
Hence repulsion between bond pairs in n f 3, is less than n h 3. As we move down the group, radius of elements increases and electronegativity decreases so. As water has two lone pairs the. As a result the bond angle decreases. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about.
Trigonal Pyramidal Bond Angle
As water has two lone pairs the. Hence repulsion between bond pairs in n f 3, is less than n h 3. As a result the bond angle decreases. As we move down the group, radius of elements increases and electronegativity decreases so. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about.
ii) Bond angle of NH3 is than H2O. Justify
Hence repulsion between bond pairs in n f 3, is less than n h 3. As we move down the group, radius of elements increases and electronegativity decreases so. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As water has two lone pairs the. As a result the bond.
NH3 Molecular Geometry, Hybridization, Bond Angle and Molecular Shape
As a result the bond angle decreases. Hence repulsion between bond pairs in n f 3, is less than n h 3. As we move down the group, radius of elements increases and electronegativity decreases so. As water has two lone pairs the. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about.
Decreasing order of bond angle of (NH3 ,PH3 ,AsH3 ) Filo
As we move down the group, radius of elements increases and electronegativity decreases so. Hence repulsion between bond pairs in n f 3, is less than n h 3. As water has two lone pairs the. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As a result the bond.
Why nh3 has more bond angle than nf3 Chemistry Chemical Bonding and
As water has two lone pairs the. Hence repulsion between bond pairs in n f 3, is less than n h 3. As we move down the group, radius of elements increases and electronegativity decreases so. As a result the bond angle decreases. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about.
SOLVED Bond angle in nh3 is greater than bond angle in a s h 3
As a result the bond angle decreases. As we move down the group, radius of elements increases and electronegativity decreases so. Hence repulsion between bond pairs in n f 3, is less than n h 3. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As water has two lone.
As Water Has Two Lone Pairs The.
As a result the bond angle decreases. So, the bond angle in n h 3 is less than 109.5 ∘ which i s about 107 ∘. As we move down the group, radius of elements increases and electronegativity decreases so. Hence repulsion between bond pairs in n f 3, is less than n h 3.