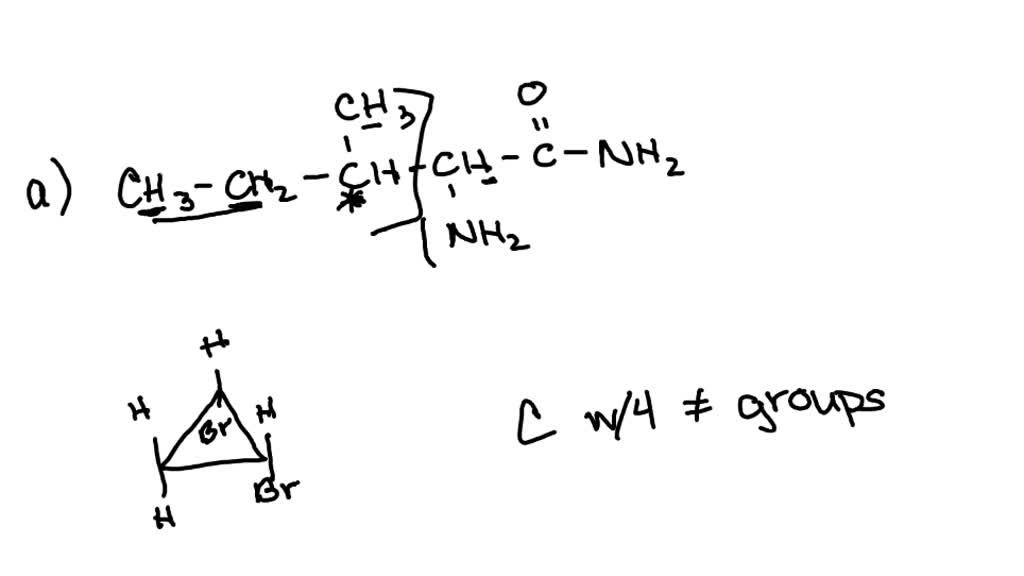What Is An Asymmetric Carbon
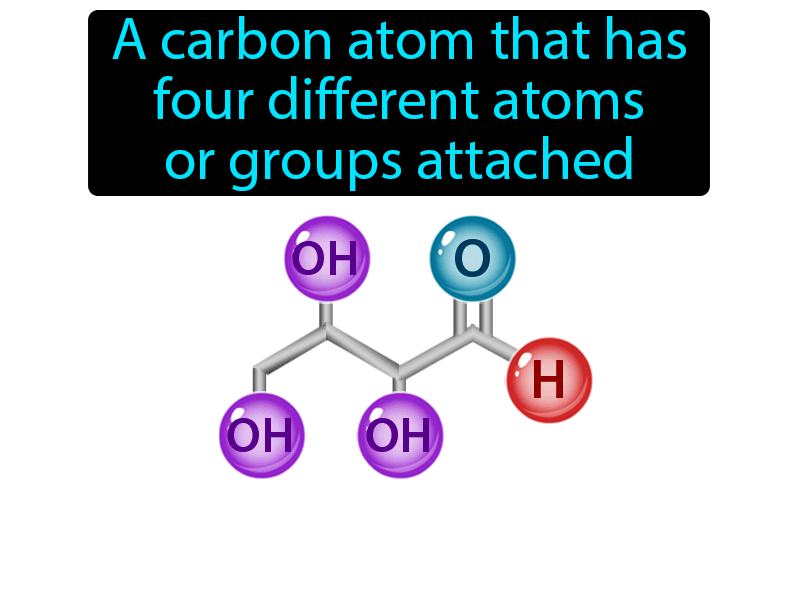
What Is An Asymmetric Carbon - An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. The leaving group is then pushed off. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. It is marked with an asterisk. No of asymmetric carbon atoms in. Α − d − g l u c o s e contains 5 asymmetric carbons.
No of asymmetric carbon atoms in. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. Α − d − g l u c o s e contains 5 asymmetric carbons. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. It is marked with an asterisk. The leaving group is then pushed off.
It is marked with an asterisk. The leaving group is then pushed off. Α − d − g l u c o s e contains 5 asymmetric carbons. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. No of asymmetric carbon atoms in.
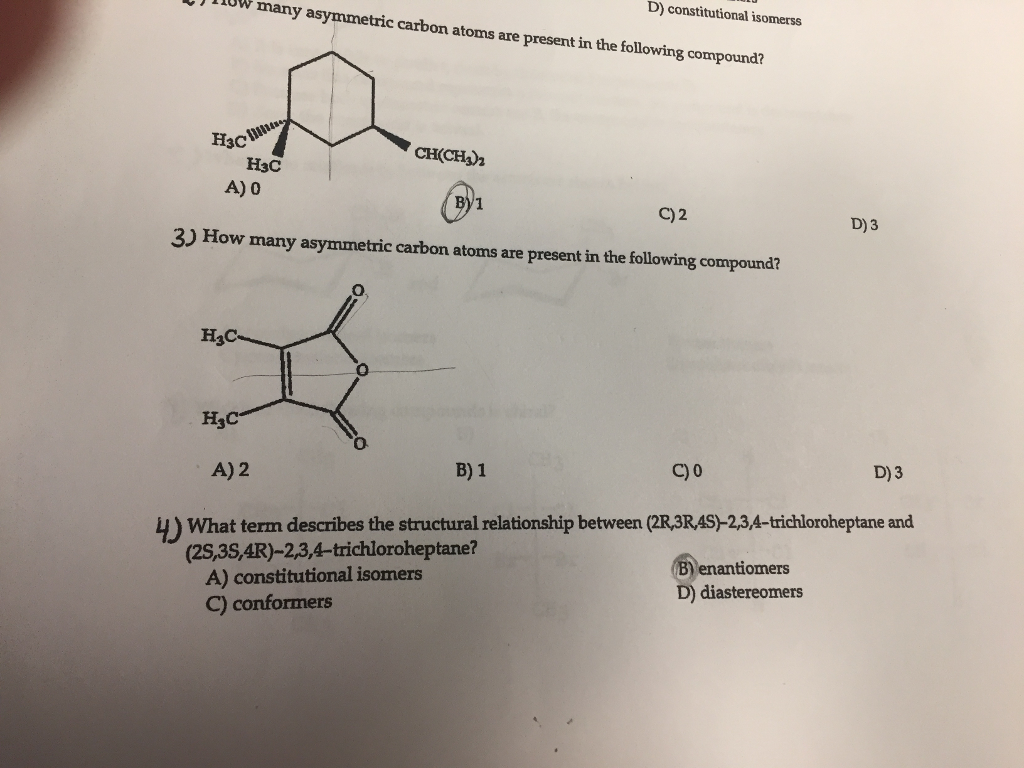
Solved How many asymmetric carbon atoms are present in the
Α − d − g l u c o s e contains 5 asymmetric carbons. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. It is marked with an asterisk. A carbon atom that is bonded to four different groups is an asymmetric.
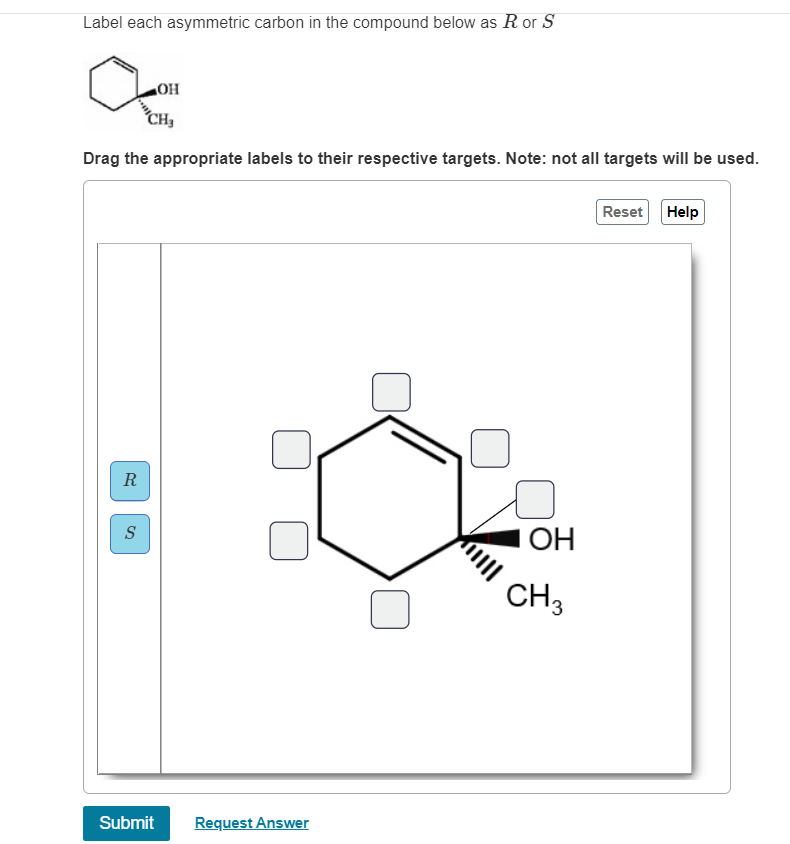
34 Label Each Asymmetric Carbon In The Compound Below As R Or S
An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. It is marked with an asterisk. The leaving group.
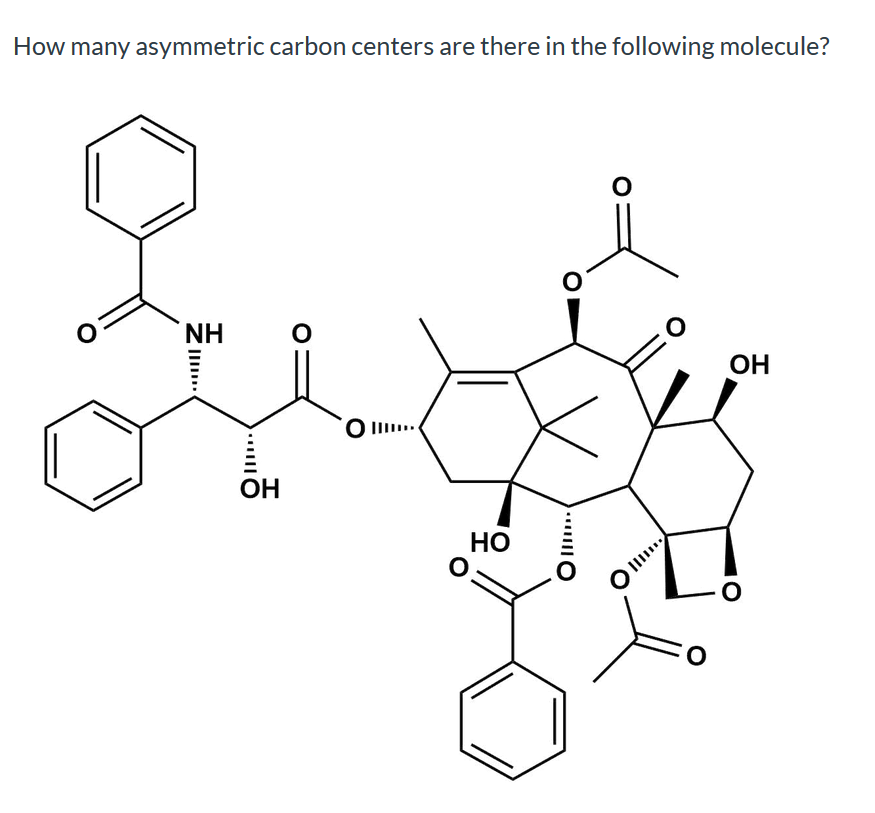
Solved How many asymmetric carbon centers are there in the
The leaving group is then pushed off. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. An asymmetric carbon atom is defined as a carbon within an organic compound that contains.
SOLVEDVISUAL SKILLS Which of the molecules shown in question 5 has an
The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. Α − d − g l u c o s e contains 5 asymmetric carbons. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state.
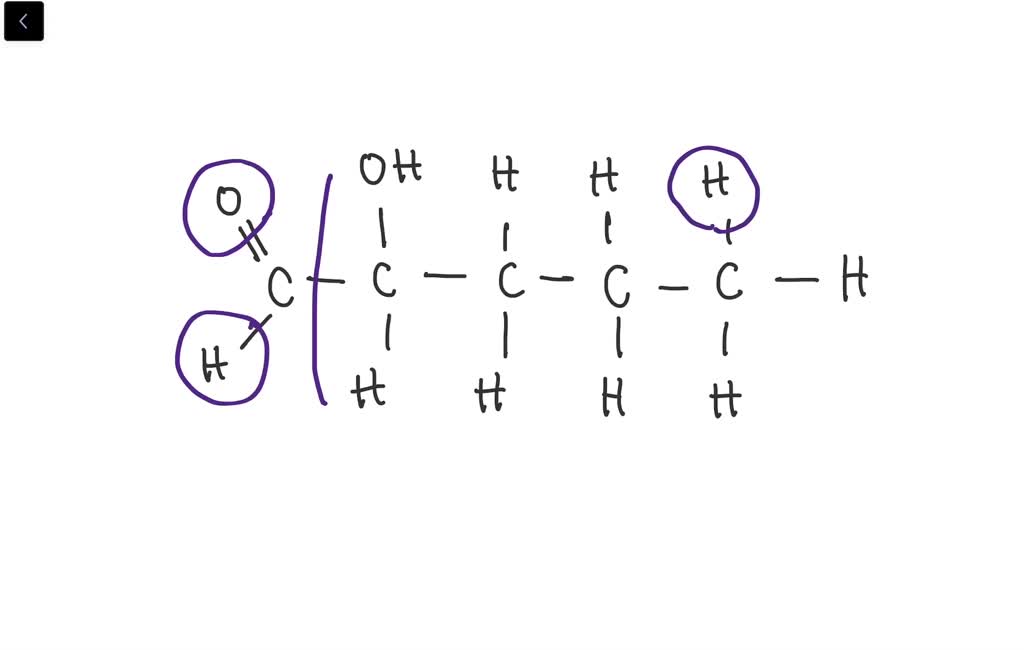
SOLVEDIndicate the asymmetric carbon atoms in the following compounds
The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. No of asymmetric carbon atoms in. A carbon atom that is bonded to four different groups is an asymmetric carbon atom..
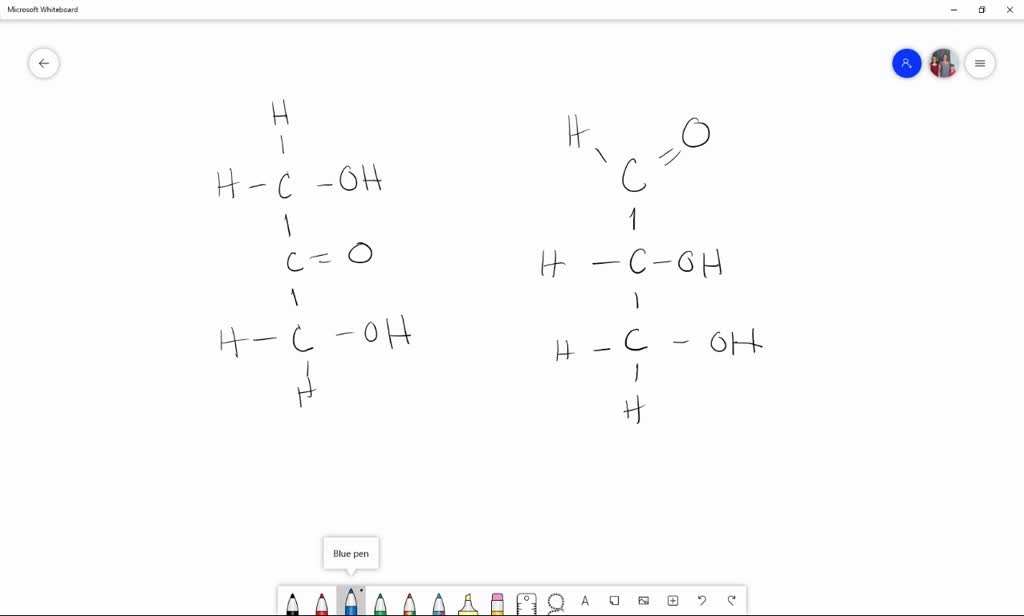
How many asymmetric carbon atoms are present in D(+) glucose
No of asymmetric carbon atoms in. It is marked with an asterisk. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. The leaving group is then pushed off. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon.
Solved How many asymmetric carbon atoms are present in the
The leaving group is then pushed off. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. No of asymmetric carbon atoms in. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. It is marked with an asterisk.
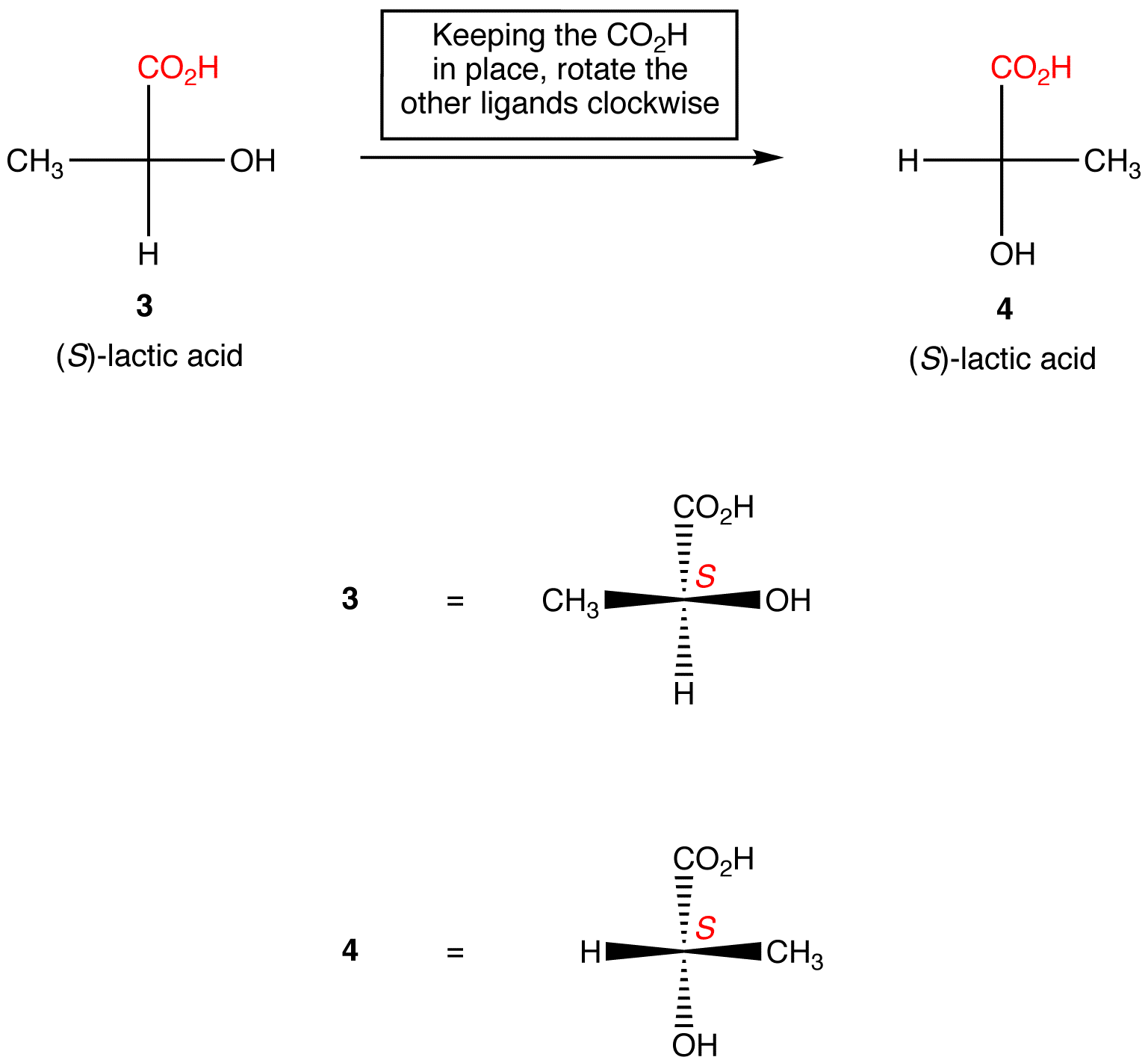
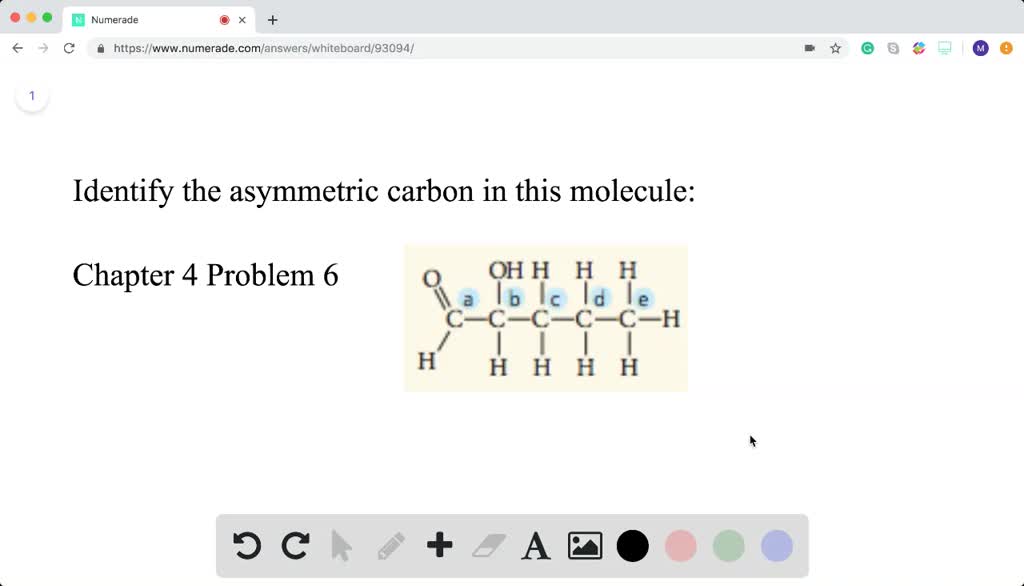
Identify the asymmetric carbon in this molecule Numerade
The leaving group is then pushed off. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through.
Visual. SKILLS Identify the asymmetric carbon in this molecule Numerade
The carbon atom which is bonded to four different atoms/groups is asymmetric carbon. Α − d − g l u c o s e contains 5 asymmetric carbons. It is marked with an asterisk. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. The leaving group is then pushed off.
Asymmetric Carbon Definition & Image GameSmartz
The leaving group is then pushed off. S n 2 reaction at asymmetric carbon means the nucleophilic substitution through the formation of transition state occurs at the chiral carbon in the compound. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. No of.
The Leaving Group Is Then Pushed Off.
No of asymmetric carbon atoms in. An asymmetric carbon atom is defined as a carbon within an organic compound that contains four different atoms or groups of atoms (substituents) bonded to it. A carbon atom that is bonded to four different groups is an asymmetric carbon atom. The carbon atom which is bonded to four different atoms/groups is asymmetric carbon.
S N 2 Reaction At Asymmetric Carbon Means The Nucleophilic Substitution Through The Formation Of Transition State Occurs At The Chiral Carbon In The Compound.
Α − d − g l u c o s e contains 5 asymmetric carbons. It is marked with an asterisk.