What Happens When Sodium Reacts With Chlorine
What Happens When Sodium Reacts With Chlorine - When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. This electron is then gained by the chlorine atom which becomes a negative ion. As this happens, the electron is transferred from. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) The sodium atom loses an electron to become a positive ion. This is an example of ionic bonding. Sodium and chloride form an ionic bond. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron.
This is an example of ionic bonding. Sodium and chloride form an ionic bond. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) This electron is then gained by the chlorine atom which becomes a negative ion. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. The sodium atom loses an electron to become a positive ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. As this happens, the electron is transferred from.
This electron is then gained by the chlorine atom which becomes a negative ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. Sodium and chloride form an ionic bond. The sodium atom loses an electron to become a positive ion. This is an example of ionic bonding. As this happens, the electron is transferred from. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas.
Solved 1. Sodium metal reacts violently with chlorine gas to
Sodium and chloride form an ionic bond. The sodium atom loses an electron to become a positive ion. As this happens, the electron is transferred from. This is an example of ionic bonding. 👍 helpful ( 0 ) 👎 not helpful ( 0 )
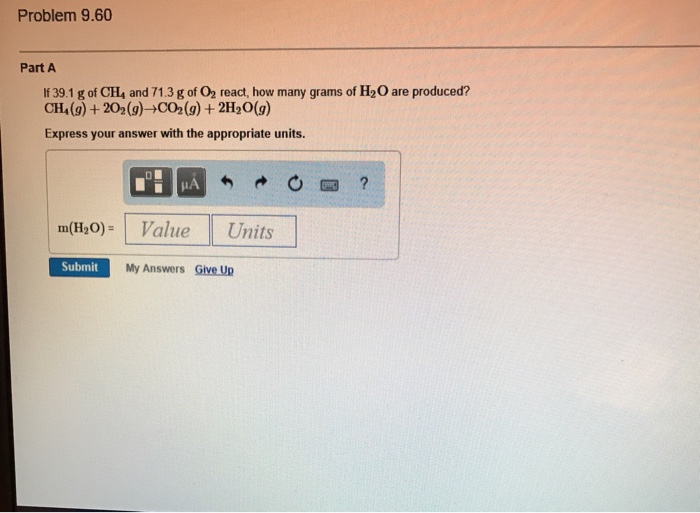
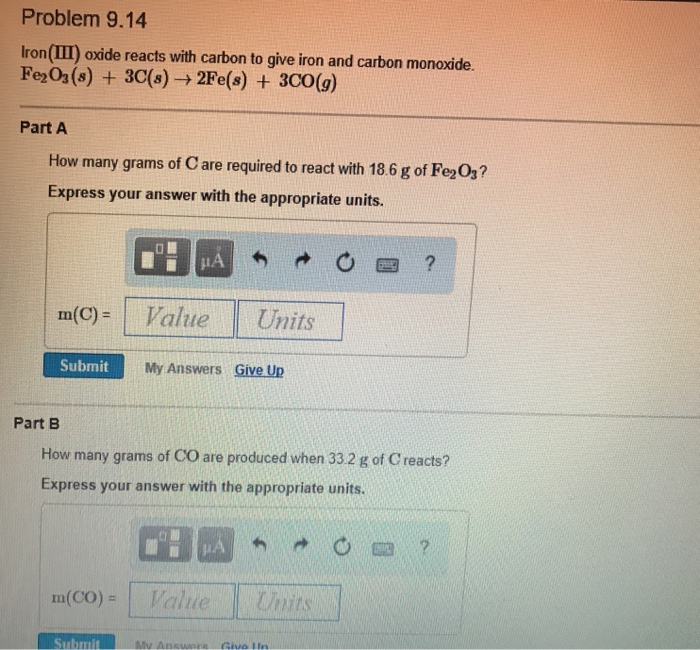
Solved Part A A sample of sodium reacts completely with
As this happens, the electron is transferred from. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. Sodium and chloride form an ionic bond. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron..
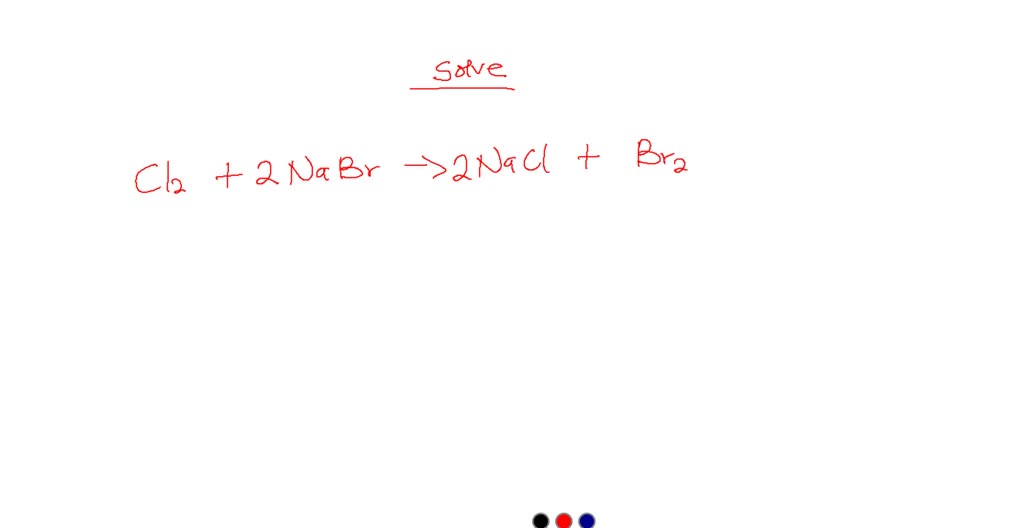
When chlorine gas is bubbled into a solution of sodium bromide, the
👍 helpful ( 0 ) 👎 not helpful ( 0 ) This electron is then gained by the chlorine atom which becomes a negative ion. Sodium and chloride form an ionic bond. This is an example of ionic bonding. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable.
SOLVED What happens when (4)a. Magnesium reacts with dilute HCl?b
This is an example of ionic bonding. This electron is then gained by the chlorine atom which becomes a negative ion. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. Therefore the sodium atom loses one electron from its outer shell and.
Chlorine reacts with sodium to form the compound NaCl. Chlorine also reac..
This electron is then gained by the chlorine atom which becomes a negative ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. This.
Solved Part A A sample of sodium reacts completely with
The sodium atom loses an electron to become a positive ion. This electron is then gained by the chlorine atom which becomes a negative ion. As this happens, the electron is transferred from. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas..
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce
This electron is then gained by the chlorine atom which becomes a negative ion. This is an example of ionic bonding. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) The sodium atom loses an electron to become a positive ion. Sodium and chloride form an ionic bond.
SOLVEDDraw a diagram to show what happens when a sodium atom reacts
Sodium and chloride form an ionic bond. This electron is then gained by the chlorine atom which becomes a negative ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. As this happens, the electron is transferred from.
SOLVEDSodium reacts with chlorine gas according to the reaction 2 Na
Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. This electron is then gained by the chlorine atom which becomes a negative ion. The sodium atom loses an electron to become a positive ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) As this happens, the electron is.
Solved Part A A sample of sodium reacts completely with
This electron is then gained by the chlorine atom which becomes a negative ion. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) This is an example of ionic bonding. When sodium reacts with chlorine, the sodium atom transfers 1 electron to the chlorine atom, in order to achieve a stable electron configuration resembling a noble gas. As.
When Sodium Reacts With Chlorine, The Sodium Atom Transfers 1 Electron To The Chlorine Atom, In Order To Achieve A Stable Electron Configuration Resembling A Noble Gas.
This electron is then gained by the chlorine atom which becomes a negative ion. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. 👍 helpful ( 0 ) 👎 not helpful ( 0 ) Sodium and chloride form an ionic bond.
The Sodium Atom Loses An Electron To Become A Positive Ion.
As this happens, the electron is transferred from. This is an example of ionic bonding.